Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents
Abstract
:1. Introduction
2. Results
2.1. Biochemical Composition of Extracts
2.2. Antioxidant Capacity of Lipidic Extracts
2.3. Antitumoral Features of Lipidic Extracts
2.3.1. Evaluation of Cancer Cell Viability by Sulforhodamine B Assay
2.3.2. Evaluation of Cancer Cell Death via TUNEL Assay
2.3.3. Evaluation of Cancer Cell Proliferation
2.4. Anti-Inflammatory Potential of Lipidic Extracts
2.4.1. Human Red Blood Cell (HRBC) Membrane Stabilization Assay
2.4.2. Cox Human Inhibitory Assay
2.5. Cytotoxicity
3. Discussion
3.1. Antioxidant Capacity of Lipidic Crude Extracts
3.2. Antitumoral Features of Cyanobacterial Extracts
3.3. Anti-Inflammatory Potential of Lipidic Crude Extracts
3.4. Potential of Application of Gloeothece sp. Extracts
4. Materials and Methods
4.1. Microorganism Source and Biomass Production
4.2. Extract Preparation
4.3. Chemical Characterization of Extracts
4.3.1. Profile and Content of Polyunsaturated Fatty Acids
4.3.2. Profile and Content of Carotenoids
4.4. Antioxidant Effects of Lipidic Extracts
4.4.1. ABTS+• Scavenging Capacity
4.4.2. DPPH• Scavenging Capacity
4.4.3. Superoxide Radical (O2•−) Scavenging Capacity
4.4.4. Nitric Oxide Radical (•NO−) Scavenging Capacity
4.5. Anticancer Effects of Gloeothece sp. Extract
4.5.1. Cancer Cell Culture
4.5.2. Cancer Cell Viability Sulforhodamine B Assay
4.5.3. Cancer Cell Death TUNEL Assay
4.5.4. Cancer Proliferative Assay
4.6. Anti-Inflammatory Effects of Extracts
4.6.1. Human Red Blood Cell (HRBC) Membrane Stabilization Assay
4.6.2. Cox Human Inhibitory Screening Assay
4.7. Cytotoxicity Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talero, E.; Garcia-Maurino, S.; Avila-Roman, J.; Rodriguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Panjiar, N.; Mishra, S.; Yadav, A.N.; Verma, D.P. Functional Foods from Cyanobacteria: An Emerging Source for Functional Food Products of Pharmaceutical Importance. In Microbial Functional Foods and Nutraceuticals, 1st ed.; Gupta, V.K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–55. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 26 January 2021).
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Díaz, P.; Valenzuela Valderrama, M.; Bravo, J.; Quest, A.F.G. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front. Microbiol. 2018, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol. Ther. 1992, 53, 127–166. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Role of Oxygen in Cancer: Looking Beyond Hypoxia. Anti-Cancer Agents Med. Chem. 2009, 9, 517–525. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58d–68d. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Li, D.; Gao, Y.; Cao, X. The Roles of Lysosomes in Inflammation and Autoimmune Diseases. Int. Rev. Immunol. 2014, 34, 415–431. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Echizen, K.; Hirose, O.; Maeda, Y.; Oshima, M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016, 107, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, A.; Andersson, M.; Lagerstedt, K.; Lonnroth, C.; Nordgren, S.; Lundholm, K. Receptor and enzyme expression for prostanoid metabolism in colorectal cancer related to tumor tissue PGE2. Int. J. Oncol. 2010, 36, 469–478. [Google Scholar]
- Shukla, Y.; George, J. Combinatorial strategies employing nutraceuticals for cancer development. Ann. N. Y. Acad. Sci. 2011, 1229, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.E. The Search for a chemoprevention agent effective against melanoma: Considerations and challenges. J. Investig. Dermatol. 2011, 131, 1401–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, J.A. Statins and the colorectum: Hope for chemoprevention? Cancer Prev. Res. 2010, 3, 573–575. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Fujita, K. Willow Leaves’ Extracts Contain Anti-Tumor Agents Effective against Three Cell Types. PLoS ONE 2007, 2, e178. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—a brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids modulate the hallmarks of cancer cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Zuluaga, M.; Gueguen, V.; Pavon-Djavid, G.; Letourneur, D. Carotenoids from microalgae to block oxidative stress. BioImpacts BI 2017, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Guermouche, B.; Soulimane-Mokhtari, N.A.; Bouanane, S.; Merzouk, H.; Merzouk, S.; Narce, M. Effect of Dietary Polyunsaturated Fatty Acids on Oxidant/Antioxidant Status in Macrosomic Offspring of Diabetic Rats. BioMed Res. Int. 2014, 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2019, 10, 602–612. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Álvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Serini, S.; Maggiano, N.; Tringali, G.; Navarra, P.; Ranelletti, F.O.; Calviello, G. beta-Carotene downregulates the steady-state and heregulin-alpha-induced COX-2 pathways in colon cancer cells. J. Nutr. 2005, 135, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, K.H.; Koo, S.Y.; Lee, D.-U. Antiproliferative Effects of Carotenoids Extracted from Chlorella ellipsoidea and Chlorella vulgaris on Human Colon Cancer Cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef]
- Sheu, M.J.; Huang, G.J.; Wu, C.H.; Chen, J.S.; Chang, H.Y.; Chang, S.J.; Chung, J.G. Ethanol extract of Dunaliella salina induces cell cycle arrest and apoptosis in A549 human non-small cell lung cancer cells. In Vivo 2008, 22, 369–378. [Google Scholar]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of Solvent System on Extractability of Lipidic Components of Scenedesmus obliquus (M2-1) and Gloeothece sp. on Antioxidant Scavenging Capacity Thereof. Mar. Drugs 2015, 13, 6453–6471. [Google Scholar] [CrossRef] [Green Version]
- Amaro, H.M.; Guedes, A.C.; Preto, M.A.C.; Sousa-Pinto, I.; Malcata, F.X. Gloeothece sp. as a Nutraceutical Source-An Improved Method of Extraction of Carotenoids and Fatty Acids. Mar. Drugs 2018, 16, 327. [Google Scholar] [CrossRef] [Green Version]
- European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, L 141, 3–11. [Google Scholar]
- Bargiela-Iparraguirre, J.; Prado-Marchal, L.; Fernandez-Fuente, M.; Gutierrez-Gonzalez, A.; Moreno-Rubio, J.; Munoz-Fernandez, M.; Sereno, M.; Sanchez-Prieto, R.; Perona, R.; Sanchez-Perez, I. MCHK1 expression in Gastric Cancer is modulated by p53 and RB1/E2F1: Implications in chemo/radiotherapy response. Sci. Rep. 2016, 6, 21519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Azevedo, C.M.; Afonso, C.M.; Soares, J.X.; Reis, S.; Sousa, D.; Lima, R.T.; Vasconcelos, M.H.; Pedro, M.; Barbosa, J.; Gales, L.; et al. Pyranoxanthones: Synthesis, growth inhibitory activity on human tumor cell lines and determination of their lipophilicity in two membrane models. Eur. J. Med. Chem. 2013, 69, 798–816. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Maheshwari, S.U.; Harish, G.; Raj, J.B.; Kamali, S.; Hemamalani, D.; Varma, J.B.; Reddy, C.U. Investigation of in-vitro anti-inflammatory, anti-platelet and anti-arthritic activities in the leaves of Anisomeles malabarica Linn. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 745–752. [Google Scholar]
- Murugesh, N.; Vembar, S.; Damodaran, C. Studies on erythrocyte membrane IV: In vitro haemolytic activity of oleander extract. Toxicol. Lett. 1981, 8, 33–38. [Google Scholar] [CrossRef]
- Lee, J.L.; Mukhtar, H.; Bickers, D.R.; Kopelovich, L.; Athar, M. Cyclooxygenases in the skin: Pharmacological and toxicological implications. Toxicol. Appl. Pharmacol. 2003, 192, 294–306. [Google Scholar] [CrossRef]
- Palesh, O.; Scheiber, C.; Kesler, S.; Mustian, K.; Koopman, C.; Schapira, L. Management of side effects during and post-treatment in breast cancer survivors. Breast J. 2018, 24, 167–175. [Google Scholar] [CrossRef]
- Dutot, M.; Fagon, R.; Rousseau, D.; Rat, P. Antioxidant and Anti-Inflammatory Effects of PUFA-Rich Marine Oils: Application to the Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2009, 50, 919. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef]
- He, R.-R.; Tsoi, B.; Lan, F.; Yao, N.; Yao, X.-S.; Kurihara, H. Antioxidant properties of lutein contribute to the protection against lipopolysaccharide-induced uveitis in mice. Chin. Med. 2011, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringham, J.M.; Stringham, N.T. Nitric Oxide and Lutein: Function, Performance, and Protection of Neural Tissue. Foods 2015, 4, 678–689. [Google Scholar] [CrossRef]
- Moraes, M.L.; Ribeiro, A.M.L.; Santin, E.; Klasing, K.C. Effects of conjugated linoleic acid and lutein on the growth performance and immune response of broiler chickens. Poult. Sci. 2016, 95, 237–246. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Xiang, J.; Su, L.; Tang, X. The regulation of nitric oxide in tumor progression and therapy. J. Int. Med. Res. 2020, 48, 0300060520905985. [Google Scholar] [CrossRef] [Green Version]
- Christodoulou, M.I.; Kontos, C.K.; Halabalaki, M.; Skaltsounis, A.L.; Scorilas, A. Nature promises new anticancer agents: Interplay with the apoptosis-related BCL2 gene family. Anticancer Agents Med. Chem. 2014, 14, 375–399. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, J.H.; Kim, J.S.; Kim, M.K.; Aruoma, O.I.; Sung, M.K. Effects of quercetin and beta-carotene supplementation on azoxymethane-induced colon carcinogenesis and inflammatory responses in rats fed with high-fat diet rich in omega-6 fatty acids. Biofactors 2006, 27, 137–146. [Google Scholar] [CrossRef]
- Bie, N.; Han, L.; Meng, M.; Zhang, Y.; Guo, M.; Wang, C. Anti-tumor mechanism of eicosapentaenoic acid (EPA) on ovarian tumor model by improving the immunomodulatory activity in F344 rats. J. Funct. Foods 2020, 65, 103739. [Google Scholar] [CrossRef]
- Biondo, P.D.; Brindley, D.N.; Sawyer, M.B.; Field, C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008, 19, 787–796. [Google Scholar] [CrossRef]
- Kwon, J.I.; Kim, G.Y.; Park, K.Y.; Ryu, C.H.; Choi, Y.H. Induction of apoptosis by linoleic acid is associated with the modulation of Bcl-2 family and Fas/FasL system and activation of caspases in AGS human gastric adenocarcinoma cells. J. Med. Food 2008, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Linoleic Acid-Induced Growth Inhibition of Human Gastric Epithelial Adenocarcinoma AGS Cells is Associated with Down-Regulation of Prostaglandin E2 Synthesis and Telomerase Activity. J. Cancer Prev. 2014, 19, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef] [Green Version]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory Effects of Violaxanthin Isolated from Microalga Chlorella ellipsoidea in RAW 264.7 Macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Mazhar, D.; Ang, R.; Waxman, J. COX inhibitors and breast cancer. Br. J. Cancer 2006, 94, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [Green Version]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Cohen, Z.; Vonshak, A.; Richmond, A. Effect on environmental conditions on fatty acids composition of the red alga Phorphyridium cruentum: Correlation to growth rate. J. Phycol. 1988, 24, 328–332. [Google Scholar] [CrossRef]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentao, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
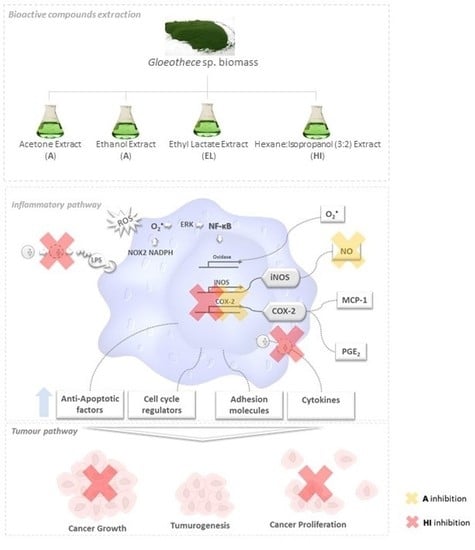
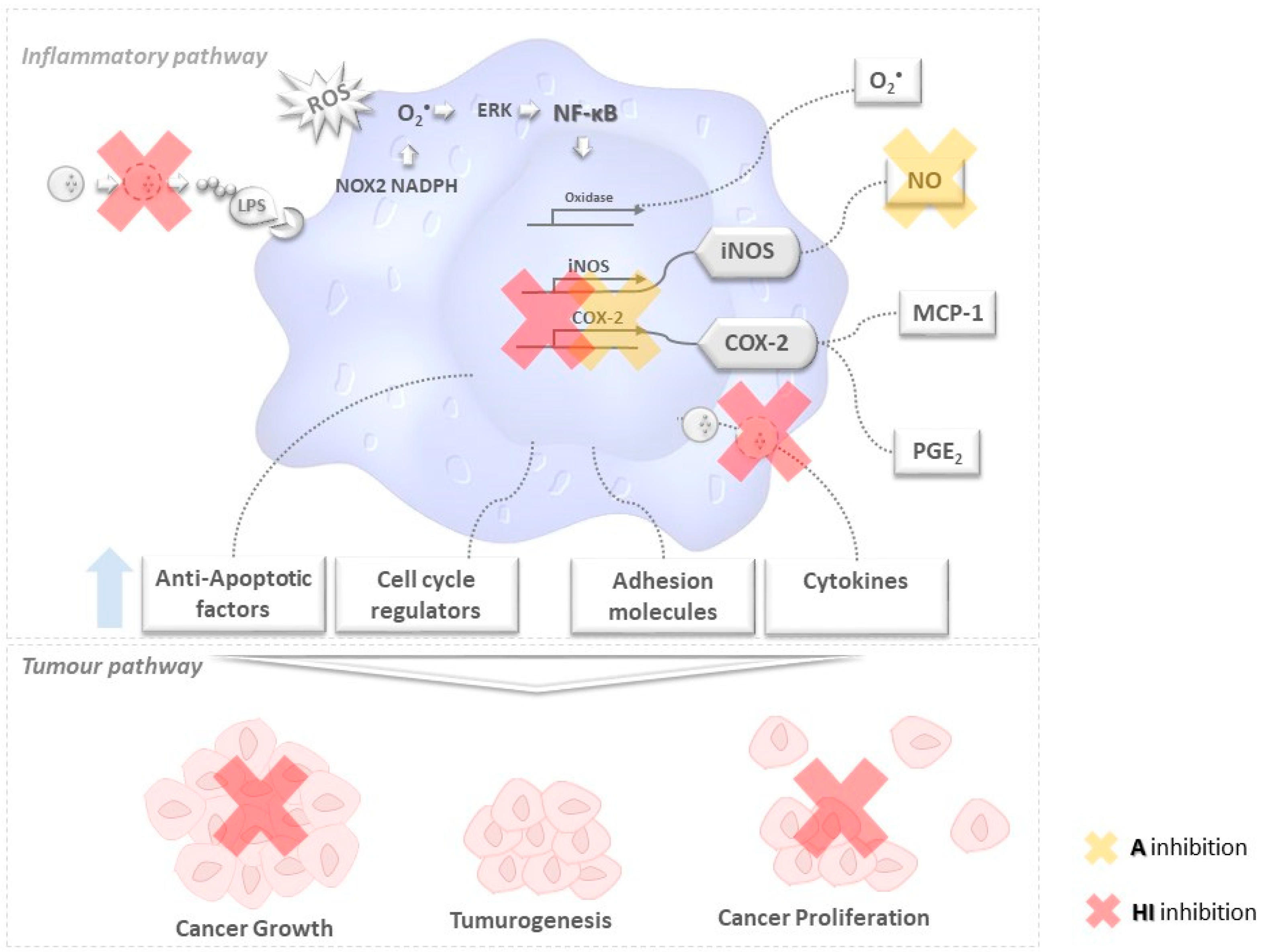
 ) secrete or degrade inflammatory cytokines in regulating cytokines released by immune cells through a feedback mechanism. Phosphorylation of NF-κB activates several enzymes, e.g., cyclooxygenase(COX-2), oxidase, and iNOS, thus inducing the release of prostaglandins (PGE2), O2•− and other molecules like anti-apoptotic factors, cell cycle regulators, adhesion molecules that are likely to be related to tumorogenesis, cancer cell growth and proliferation. The unbalanced increase of the former may lead to tumorogenesis and (among other events) cancer growth and proliferation.
) secrete or degrade inflammatory cytokines in regulating cytokines released by immune cells through a feedback mechanism. Phosphorylation of NF-κB activates several enzymes, e.g., cyclooxygenase(COX-2), oxidase, and iNOS, thus inducing the release of prostaglandins (PGE2), O2•− and other molecules like anti-apoptotic factors, cell cycle regulators, adhesion molecules that are likely to be related to tumorogenesis, cancer cell growth and proliferation. The unbalanced increase of the former may lead to tumorogenesis and (among other events) cancer growth and proliferation.
 ) secrete or degrade inflammatory cytokines in regulating cytokines released by immune cells through a feedback mechanism. Phosphorylation of NF-κB activates several enzymes, e.g., cyclooxygenase(COX-2), oxidase, and iNOS, thus inducing the release of prostaglandins (PGE2), O2•− and other molecules like anti-apoptotic factors, cell cycle regulators, adhesion molecules that are likely to be related to tumorogenesis, cancer cell growth and proliferation. The unbalanced increase of the former may lead to tumorogenesis and (among other events) cancer growth and proliferation.
) secrete or degrade inflammatory cytokines in regulating cytokines released by immune cells through a feedback mechanism. Phosphorylation of NF-κB activates several enzymes, e.g., cyclooxygenase(COX-2), oxidase, and iNOS, thus inducing the release of prostaglandins (PGE2), O2•− and other molecules like anti-apoptotic factors, cell cycle regulators, adhesion molecules that are likely to be related to tumorogenesis, cancer cell growth and proliferation. The unbalanced increase of the former may lead to tumorogenesis and (among other events) cancer growth and proliferation.
 MUFA,
MUFA,  PUFA,
PUFA,  carotenoids,
carotenoids,  phenolic compounds, and other unidentified compounds, obtained with acetone (A), ethanol (E), hexane:isopropanol (1:1, v/v) (HI) and ethyl lactate (EL).
phenolic compounds, and other unidentified compounds, obtained with acetone (A), ethanol (E), hexane:isopropanol (1:1, v/v) (HI) and ethyl lactate (EL).
 MUFA,
MUFA,  PUFA,
PUFA,  carotenoids,
carotenoids,  phenolic compounds, and other unidentified compounds, obtained with acetone (A), ethanol (E), hexane:isopropanol (1:1, v/v) (HI) and ethyl lactate (EL).
phenolic compounds, and other unidentified compounds, obtained with acetone (A), ethanol (E), hexane:isopropanol (1:1, v/v) (HI) and ethyl lactate (EL).
 Acetone (A),
Acetone (A),  Ethanol (E)
Ethanol (E)  Hexane:Isopropanol (3:2) (HI) and
Hexane:Isopropanol (3:2) (HI) and  Ethyl Lactate (EL) extracts.
Ethyl Lactate (EL) extracts.
 Acetone (A),
Acetone (A),  Ethanol (E)
Ethanol (E)  Hexane:Isopropanol (3:2) (HI) and
Hexane:Isopropanol (3:2) (HI) and  Ethyl Lactate (EL) extracts.
Ethyl Lactate (EL) extracts.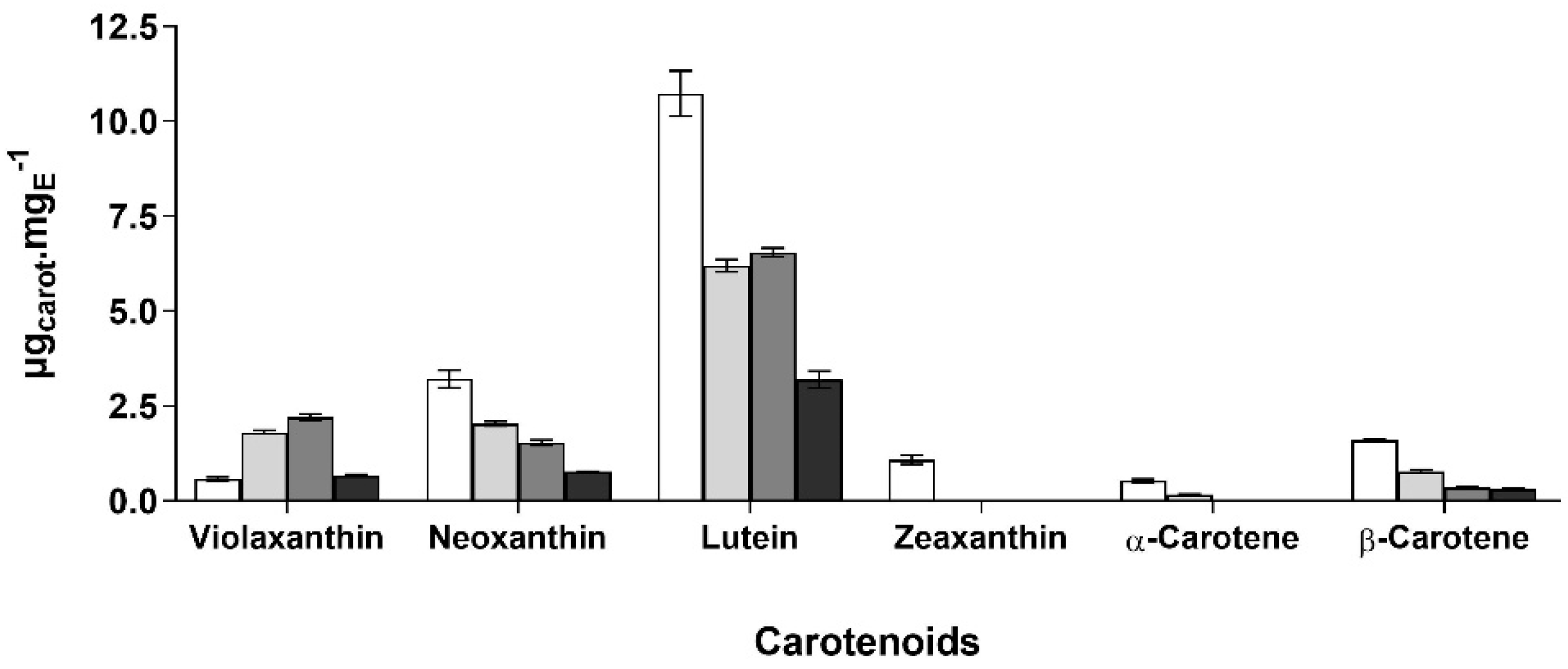
 Acetone (A),
Acetone (A),  Ethanol (E),
Ethanol (E),  hexane:isopropanol (3:2) (HI) and
hexane:isopropanol (3:2) (HI) and  Ethylic lactate (EL) extracts; and, (B) AGS cell proliferation, quantified by fold increase by Acetone (A), Ethanol (E), hexane:isopropanol (3:2) (HI) and Ethylic lactate (EL) extracts, using DMSO as a negative control. Bars with a common character are significantly not different (p < 0.05) from the DMSO control.
Ethylic lactate (EL) extracts; and, (B) AGS cell proliferation, quantified by fold increase by Acetone (A), Ethanol (E), hexane:isopropanol (3:2) (HI) and Ethylic lactate (EL) extracts, using DMSO as a negative control. Bars with a common character are significantly not different (p < 0.05) from the DMSO control.
 Acetone (A),
Acetone (A),  Ethanol (E),
Ethanol (E),  hexane:isopropanol (3:2) (HI) and
hexane:isopropanol (3:2) (HI) and  Ethylic lactate (EL) extracts; and, (B) AGS cell proliferation, quantified by fold increase by Acetone (A), Ethanol (E), hexane:isopropanol (3:2) (HI) and Ethylic lactate (EL) extracts, using DMSO as a negative control. Bars with a common character are significantly not different (p < 0.05) from the DMSO control.
Ethylic lactate (EL) extracts; and, (B) AGS cell proliferation, quantified by fold increase by Acetone (A), Ethanol (E), hexane:isopropanol (3:2) (HI) and Ethylic lactate (EL) extracts, using DMSO as a negative control. Bars with a common character are significantly not different (p < 0.05) from the DMSO control.
 50,
50,  100,
100,  200 and
200 and  300 μg·mL−1, by 24 h (A) and 48 h (B). Bars marked with the same letter in the same superscript have no significant difference relative to the control (p < 0.05).
300 μg·mL−1, by 24 h (A) and 48 h (B). Bars marked with the same letter in the same superscript have no significant difference relative to the control (p < 0.05).
 50,
50,  100,
100,  200 and
200 and  300 μg·mL−1, by 24 h (A) and 48 h (B). Bars marked with the same letter in the same superscript have no significant difference relative to the control (p < 0.05).
300 μg·mL−1, by 24 h (A) and 48 h (B). Bars marked with the same letter in the same superscript have no significant difference relative to the control (p < 0.05).
| Fatty Acids | Fatty Acids Concentration and Content (μgFA·mgE−1, %(mFA/mTFA) | |||
|---|---|---|---|---|
| E | A | HI (3:2) | EL | |
| C14:1 | 0.520 ± 0.002 | 1.495 ± 0.013 | 0.937 ± 0.001 | 0.607 ± 0.020 |
| 0.3 | 1.3 | 2.3 | 3.3 | |
| C16:1 | 1.046 ± 0.053 | 2.426 ± 0.158 | 0.994 ± 0.023 | 0.869 ± 0.092 |
| 0.7 | 1.7 | 2.7 | 3.7 | |
| C17:1 | 6.849 ± 0.012 | 19.154 ± 2.152 | 2.517 ± 0.099 | 1.017 ± 0.187 |
| 4.3 | 5.3 | 6.3 | 7.3 | |
| C18:1 n9 c+t | 22.812 ± 1.118 | 53.796 ± 2.918 | 12.910 ± 2.598 a | 12.767 ± 1.980 a |
| 14.4 | 15.4 | 16.4 | 17.4 | |
| C22:1 n9 | 0.184 ± 0.010 a | 0.849 ± 0.043 | 0.202 ± 0.057 a | 0.317 ± 0.016 |
| 0.3 | 0.2 | 2.3 | 3.3 | |
| Σ MUFA | 31.412 | 76.870 | 17.559 | 15.577 |
| 20.0 | 22.4 | 8.5 | 15.6 | |
| C18:2 n6 t | 24.242 ± 0.597 | 59.711 ± 3.278 | 11.683 ± 1.432 | 6.240 ± 1.510 |
| 15.3 | 16.3 | 17.3 | 26.4 | |
| C18:2 n6 c | 0.406 ± 0.025 | 0.984 ± 0.012 | 0.308 ± 0.083 a | 0.337 ± 0.008 a |
| 0.3 | 1.3 | 2.3 | 3.3 | |
| C18:3n6 | 1.934 ± 0.030 | 1.467 ± 0.039 a | 1.250 ± 0.152 b | 1.267 ± 0.196 a,b |
| 1.2 | 2.2 | 3.2 | 4.2 | |
| C18:3 n3 | 37.233 ± 0.685 | 96.765 ± 5.713 | 13.216 ± 0.225 | 4.575 ± 1.437 |
| 23.4 | 24.4 | 25.4 | 18.3 | |
| C20:2 | 0.498 ± 0.009 | 0.724 ± 0.205 a | 0.943 ± 0.701 a | 0.289 ± 0.014 |
| 0.25 | 1.3 | 2.3 | 3.3 | |
| C20:5 n3 | 0.344 ± 0.023 a | - | 0.283 ± 0.105 a | 0.462 ± 0.071 |
| 0.2 | - | 2.2 | 3.2 | |
| Σ PUFA | 64.160 | 159.651 | 27.682 | 13.170 |
| 40.7 | 46.0 | 11.5 | 13.2 | |
| Solvents | Antioxidant Capacity IC50 (mgE·mL−1) | SRB IC50 (µgE·mL−1) | |||
|---|---|---|---|---|---|
| ABTS•+ | DPPH• | O2•− | •NO− | ||
| Ethanol | 0.259 ± 0.074 a,b | 1.538 ± 0.012 | nd | 0.637 ± 0.024 | 241.0 ± 22.5 a |
| Acetone | 0.217 ± 0.009 a | 0.978 ± 0.032 | nd | 0.284 ± 0.090 | 114.4 ± 6.4 |
| HI 3:2 (v/v) | 0.283 ± 0.034 b | nd | nd | 1.258 ± 0.353 | 23.2 ± 1.9 |
| Ethyl lactate | 5.809 ± 0.203 | 4.016 ± 1.256 | nd | nd | 209.3 ± 11.0 a |
| Solvents | HRBC Stabilization (%) | COX-2 Enzymatic Activity Inhibition IC50 (µgE·mL−1) |
|---|---|---|
| Acetone | - | 116.8 ± 7.7 |
| Ethanol | - | 198.3 ± 15.2 |
| HI 3:2 (v/v) | 61.6 ± 9.2 | 130.2 ± 7.4 |
| Ethyl lactate | 14.8 ± 4.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, H.M.; Barros, R.; Tavares, T.; Almeida, R.; Pinto, I.S.; Malcata, F.X.; Guedes, A.C. Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents. Mar. Drugs 2021, 19, 623. https://doi.org/10.3390/md19110623
Amaro HM, Barros R, Tavares T, Almeida R, Pinto IS, Malcata FX, Guedes AC. Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents. Marine Drugs. 2021; 19(11):623. https://doi.org/10.3390/md19110623
Chicago/Turabian StyleAmaro, Helena M., Rita Barros, Tânia Tavares, Raquel Almeida, Isabel Sousa Pinto, Francisco Xavier Malcata, and Ana Catarina Guedes. 2021. "Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents" Marine Drugs 19, no. 11: 623. https://doi.org/10.3390/md19110623