Comparative Study of Sargassum fusiforme Polysaccharides in Regulating Cecal and Fecal Microbiota of High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Results
2.1. Chemical Structures of Sargassum fusiforme Polysaccharides
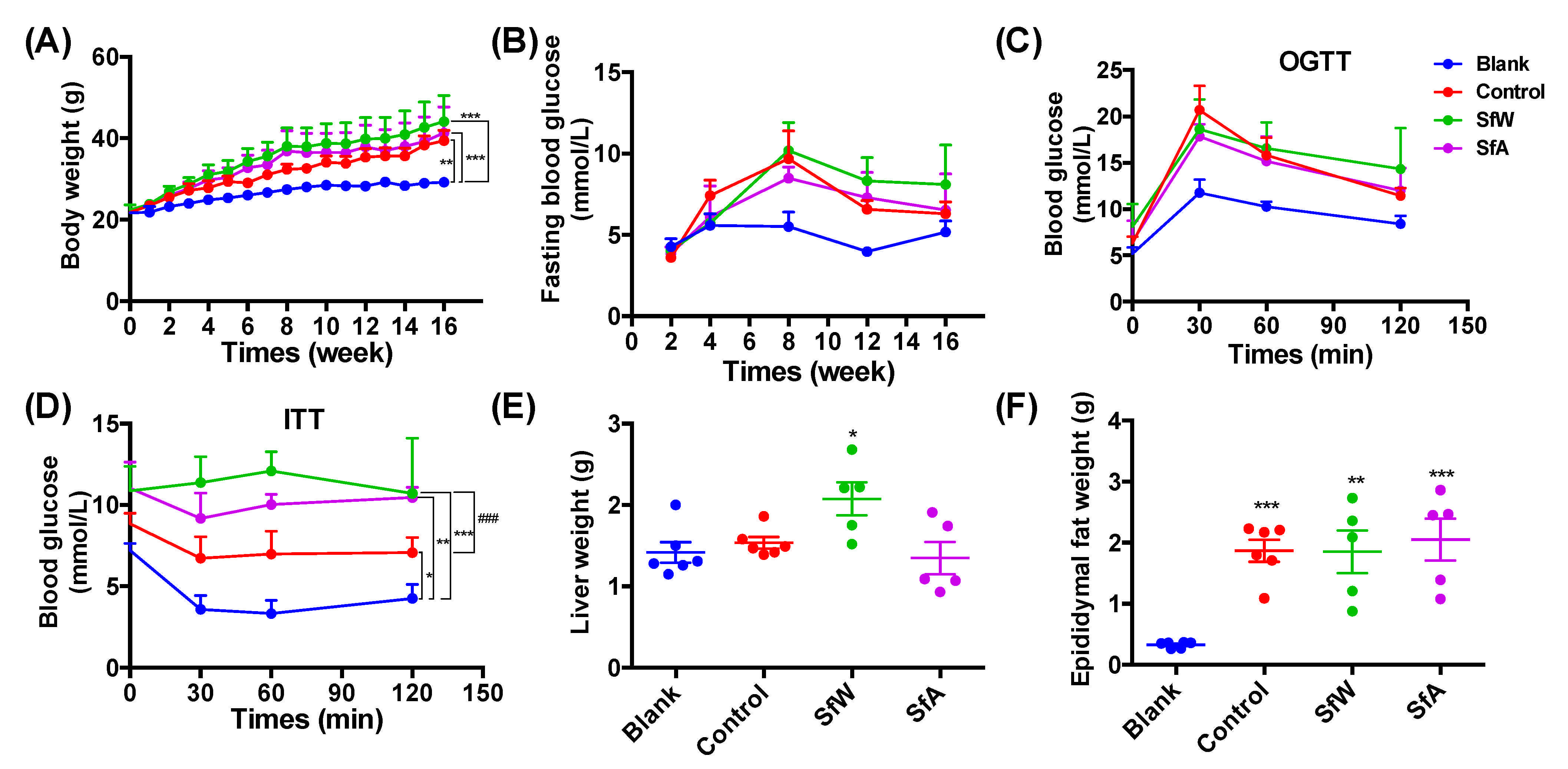
2.2. Effects of SfW and SfA on HFD-Induced Metabolic Disorders
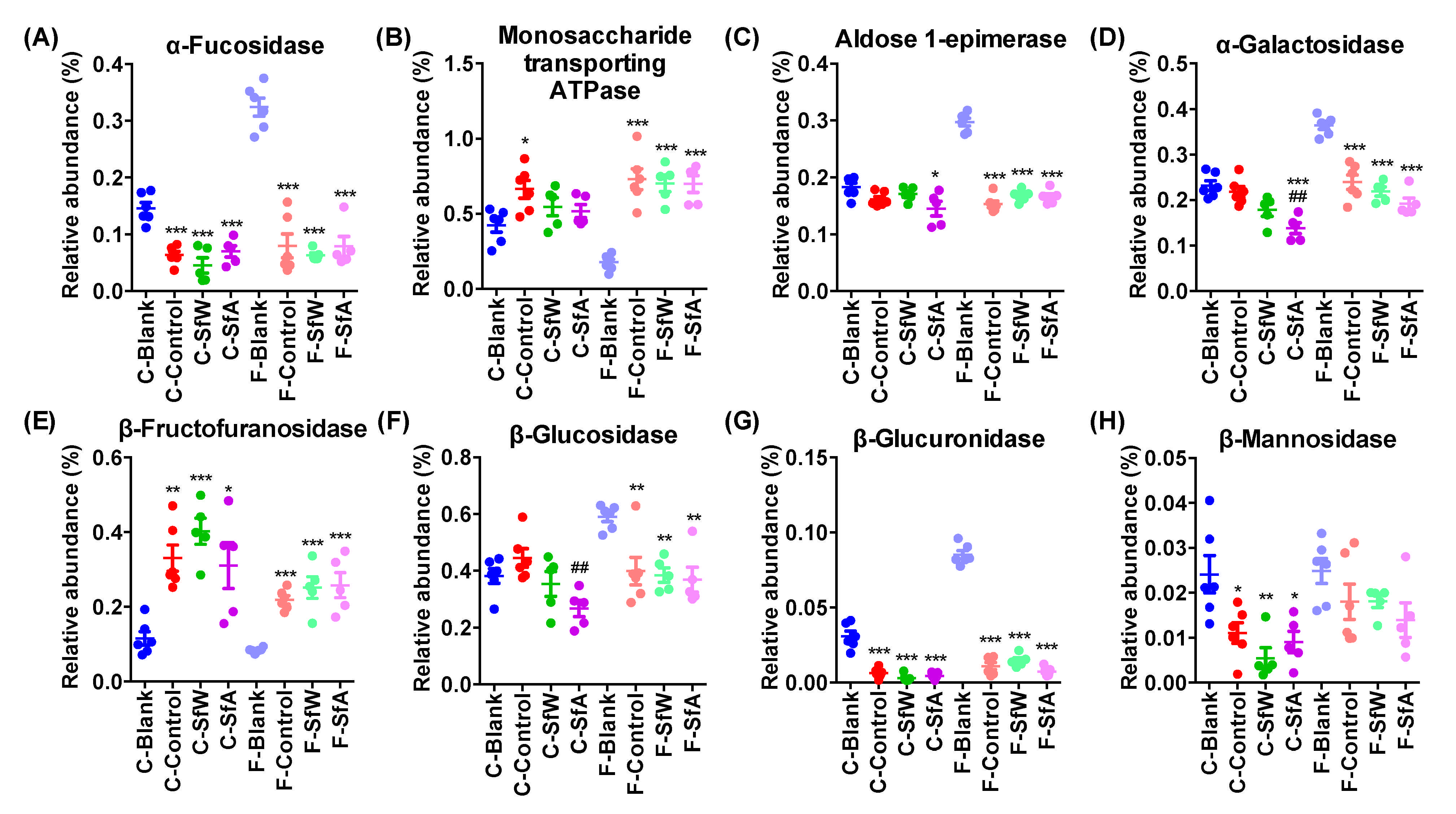
2.3. Effects of SfW and SfA on the Abundance of Genes Encoding Carbohydrate-Metabolizing Enzymes in Cecal and Fecal Microbiota
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Sargassum fusiforme Polysaccharides
4.3. Structural Analysis of Sargassum fusiforme Polysaccharides
4.4. Animal Experiments
4.5. Gut Microbiota Analysis by 16S rRNA Gene Sequencing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Valdes, A.M.; Walter, L.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ Brit. Med. J. 2018, 361, j2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, U92–U192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, R.W.; Arhire, L.; Covasa, M. Gut microbiota: From microorganisms to metabolic organ influencing obesity. Obesity 2018, 26, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Han, W.; Zhan, G.F.; Li, S.; Jiang, X.H.; Wang, L.; Xiang, S.K.; Zhu, B.; Yang, L.; Luo, A.L.; et al. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging 2019, 11, 10454–10467. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.H.; Kim, E.; Kim, S.I.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef]
- Marteau, P.; Pochart, P.; Dore, J.; Bera-Maillet, C.; Bernalier, A.; Corthier, G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl. Environ. Microb. 2001, 67, 4939–4942. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.; Geier, M.S.; Chen, H.; Hughes, R.J.; Moore, R.J. Comparison of fecal and cecal microbiotas reveals qualitative similarities but quantitative differences. BMC Microbiol. 2015, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.B.; Yang, B.; Pang, X.Y.; Chen, T.P.; Chen, F.; Cheng, K. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef]
- Shang, Q.S.; Song, G.R.; Zhang, M.F.; Shi, J.J.; Xu, C.Y.; Hao, J.J.; Li, G.Y.; Yu, G.L. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Li, S.; Li, J.H.; Mao, G.Z.; Wu, T.T.; Hu, Y.Q.; Ye, X.Q.; Tian, D.; Linhardt, R.J.; Chen, S.G. A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct. 2018, 9, 1039. [Google Scholar] [CrossRef]
- You, L.; Gong, Y.; Li, L.; Hu, X.; Brennan, C.; Kulikouskaya, V. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int. J. Food Sci. Technol. 2020, 55, 1199–1206. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, R.; You, L.; Ma, Y.; Liao, L.; Pedisić, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. Food Chem. Toxicol. 2021, 151, 112145. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum fusiforme fucoidan alleviates high-fat diet-induced obesity and insulin resistance associated with the improvement of hepatic oxidative stress and gut microbiota profile. J. Agric. Food Chem. 2020, 68, 10626–10638. [Google Scholar] [CrossRef]
- Wei, B.; Zhong, Q.W.; Ke, S.Z.; Zhou, T.S.; Xu, Q.L.; Wang, S.J.; Chen, J.W.; Zhang, H.W.; Jin, W.H.; Wang, H. Sargassum fusiforme polysaccharides prevent high-fat diet-induced early fasting hypoglycemia and regulate the gut microbiota composition. Mar. Drugs 2020, 18, 444. [Google Scholar] [CrossRef]
- Cheng, Y.; Sibusiso, L.; Hou, L.F.; Jiang, H.J.; Chen, P.C.; Zhang, X.; Wu, M.J.; Tong, H.B. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Zhou, T.S.; Qiu, W.H.; Wang, Y.K.; Xu, Q.L.; Ke, S.Z.; Wang, S.J.; Jin, W.H.; Chen, J.W.; Zhang, H.W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Wei, B.; Wang, S.J.; Ke, S.Z.; Chen, J.W.; Zhang, H.W.; Wang, H. The antioxidant activity of polysaccharides derived from marine organisms: An overview. Mar. Drugs 2019, 17, 672. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2020, 70, 1174–1182. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Zhao, A.Z. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandro, T.; Valeria, M.; Cristina, F.; Antonio, P.; Marcello, A.; Massimo, D.; Michael, S.; Sergio, U. Metaproteogenomics reveals taxonomic and functional changes between cecal and fecal microbiota in mouse. Front. Microbiol. 2017, 8, 391. [Google Scholar]
- Shahinozzaman, M.; Raychaudhuri, S.; Fan, S.; Obanda, D.N. Kale attenuates inflammation and modulates gut microbial composition and function in C57BL/6J mice with diet-induced obesity. Microorganisms 2021, 9, 238. [Google Scholar] [CrossRef]
- Kozik, A.J.; Nakatsu, C.H.; Chun, H.; Jones-Hall, Y.L. Comparison of the fecal, cecal, and mucus microbiome in male and female mice after TNBS-induced colitis. PLoS ONE 2019, 14, e0225079. [Google Scholar] [CrossRef]
- Chen, P.; Yang, S.; Hu, C.; Zhao, Z.; Wu, M. Sargassum fusiforme polysaccharide rejuvenates the small intestine in mice through altering its physiology and gut microbiota composition. Curr. Mol. Med. 2017, 17, 350–358. [Google Scholar]
- Lin, F.M.; Pomeranz, Y. Effect of borate on colorimetric determinations of carbohydrates by the phenol-sulfuric acid method. Anal. Biochem. 1968, 24, 128–131. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]


| Samples | Total Sugar (%) | Sulfate (%) | Mw (kDa) | Monosaccharide (Molar Ratio) * | |||||
|---|---|---|---|---|---|---|---|---|---|
| Man | Glc A | Glc | Gal | Xyl | Fuc | ||||
| SfW | 70.0 | 28.5 | 166/5.9 | 0.07 | 0.07 | 1.05 | 0.41 | 0.14 | 1 |
| SfA | 62.4 | 31.3 | 276/5.8 | 0.05 | 0.06 | 1.13 | 0.38 | 0.03 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, B.; Xu, Q.-L.; Zhang, B.; Zhou, T.-S.; Ke, S.-Z.; Wang, S.-J.; Wu, B.; Xu, X.-W.; Wang, H. Comparative Study of Sargassum fusiforme Polysaccharides in Regulating Cecal and Fecal Microbiota of High-Fat Diet-Fed Mice. Mar. Drugs 2021, 19, 364. https://doi.org/10.3390/md19070364
Wei B, Xu Q-L, Zhang B, Zhou T-S, Ke S-Z, Wang S-J, Wu B, Xu X-W, Wang H. Comparative Study of Sargassum fusiforme Polysaccharides in Regulating Cecal and Fecal Microbiota of High-Fat Diet-Fed Mice. Marine Drugs. 2021; 19(7):364. https://doi.org/10.3390/md19070364
Chicago/Turabian StyleWei, Bin, Qiao-Li Xu, Bo Zhang, Tao-Shun Zhou, Song-Ze Ke, Si-Jia Wang, Bin Wu, Xue-Wei Xu, and Hong Wang. 2021. "Comparative Study of Sargassum fusiforme Polysaccharides in Regulating Cecal and Fecal Microbiota of High-Fat Diet-Fed Mice" Marine Drugs 19, no. 7: 364. https://doi.org/10.3390/md19070364







