Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
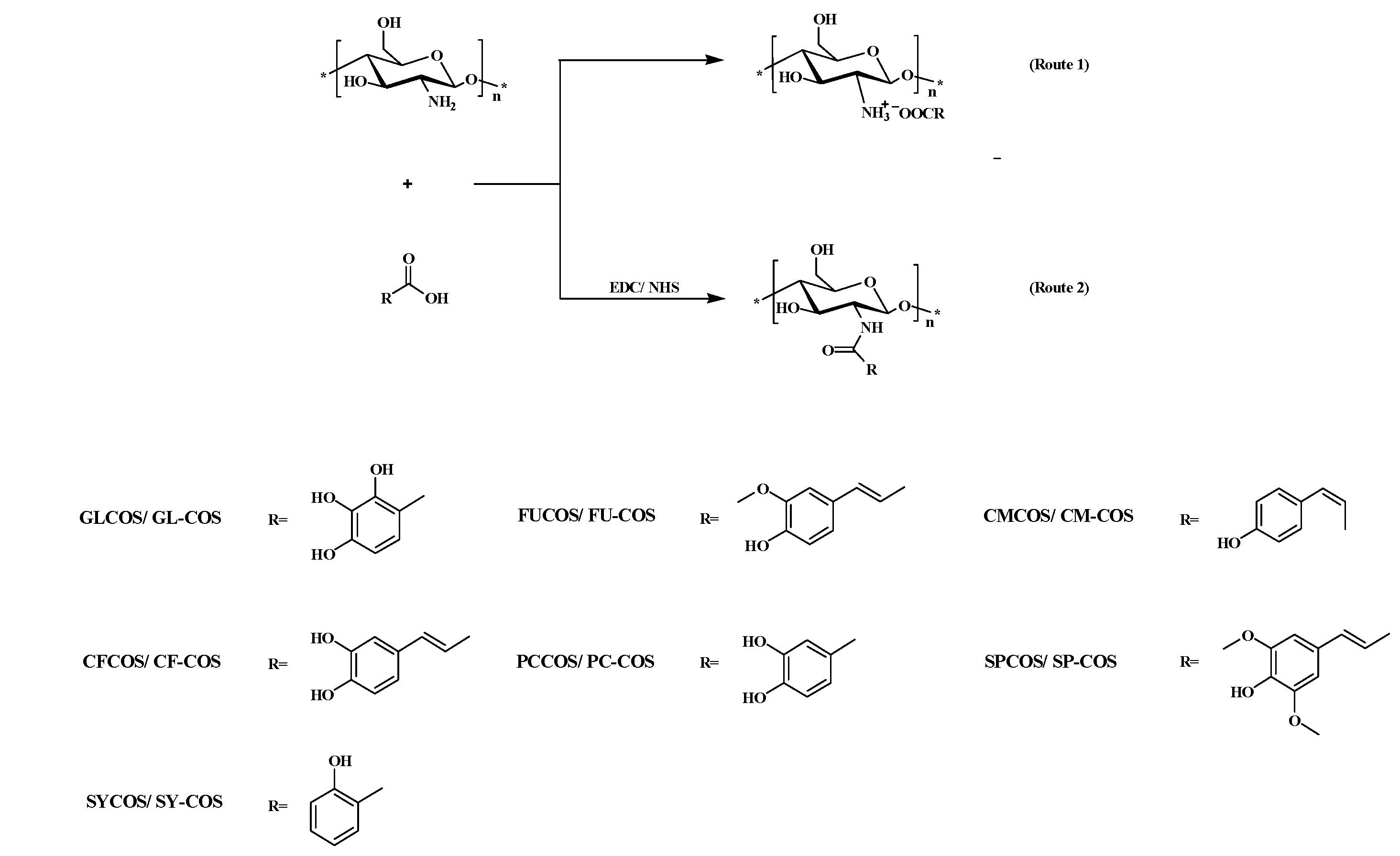
2.2. Synthesis of Chitooligosaccharide Derivatives
2.2.1. Synthesis of Phenolic Acid Chitooligosaccharide Salts
2.2.2. Synthesis of Phenolic Acid Acylated Chitooligosaccharide
2.3. Analytical Methods
2.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Antioxidant Activity Assay
2.4.1. DPPH-Radical Scavenging Activity Assay
2.4.2. Superoxide-Radical Scavenging Activity Assay
2.4.3. Hydroxyl-Radical Scavenging Activity Assay
2.4.4. Reducing Power Assay
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Synthesis and Characterization
3.1.1. FT-IR Spectra
3.1.2. 1H NMR Spectra
3.2. Antioxidant Activity
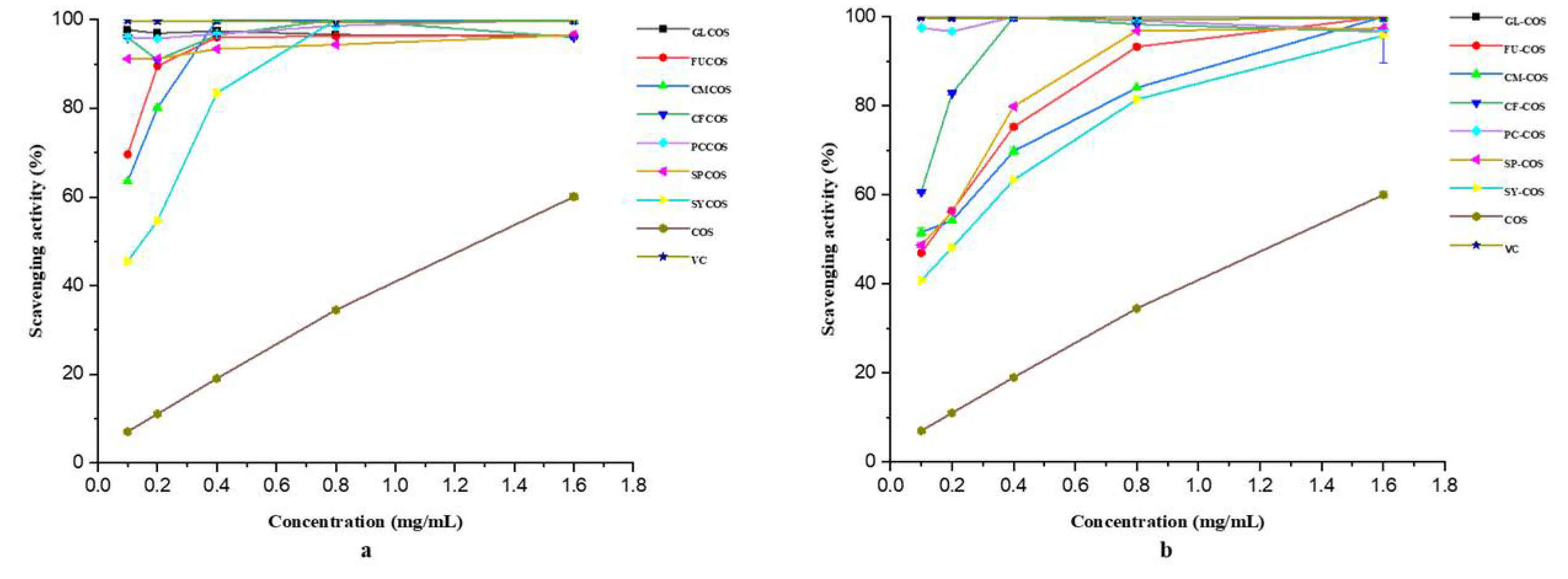
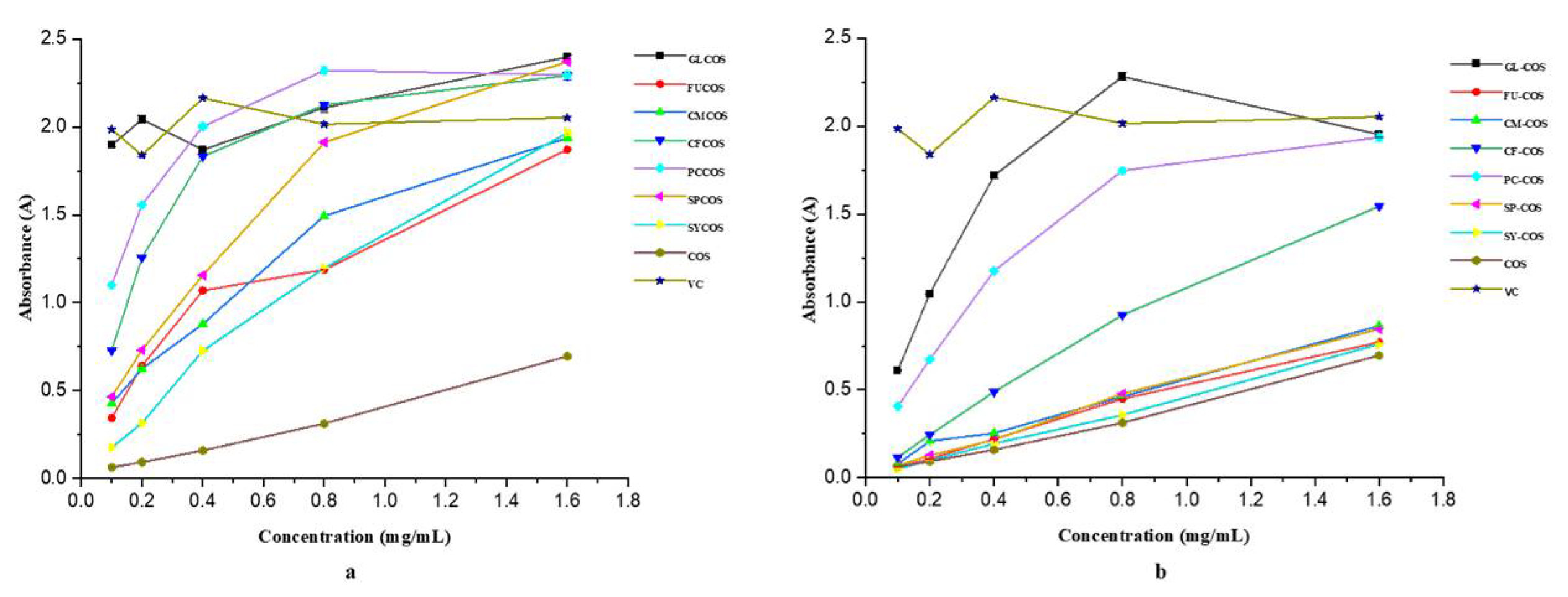
3.2.1. Scavenging Ability against DPPH Radicals
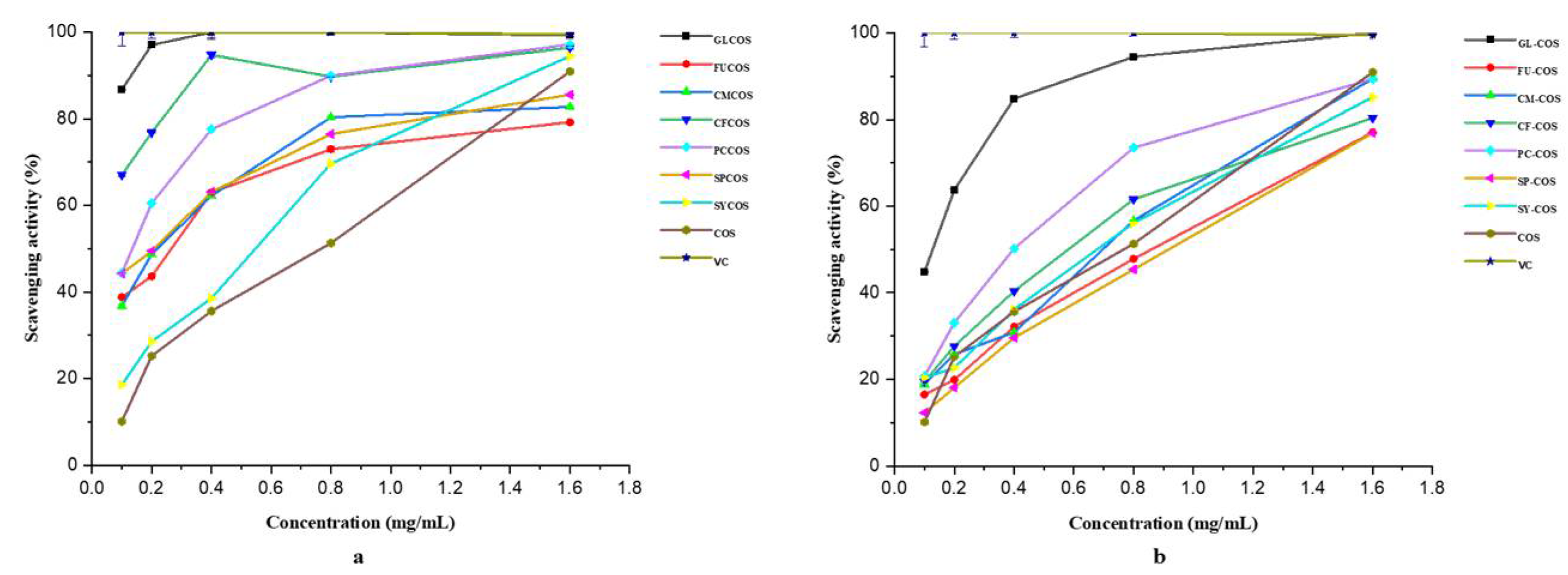
3.2.2. Scavenging Ability against Superoxide Radicals
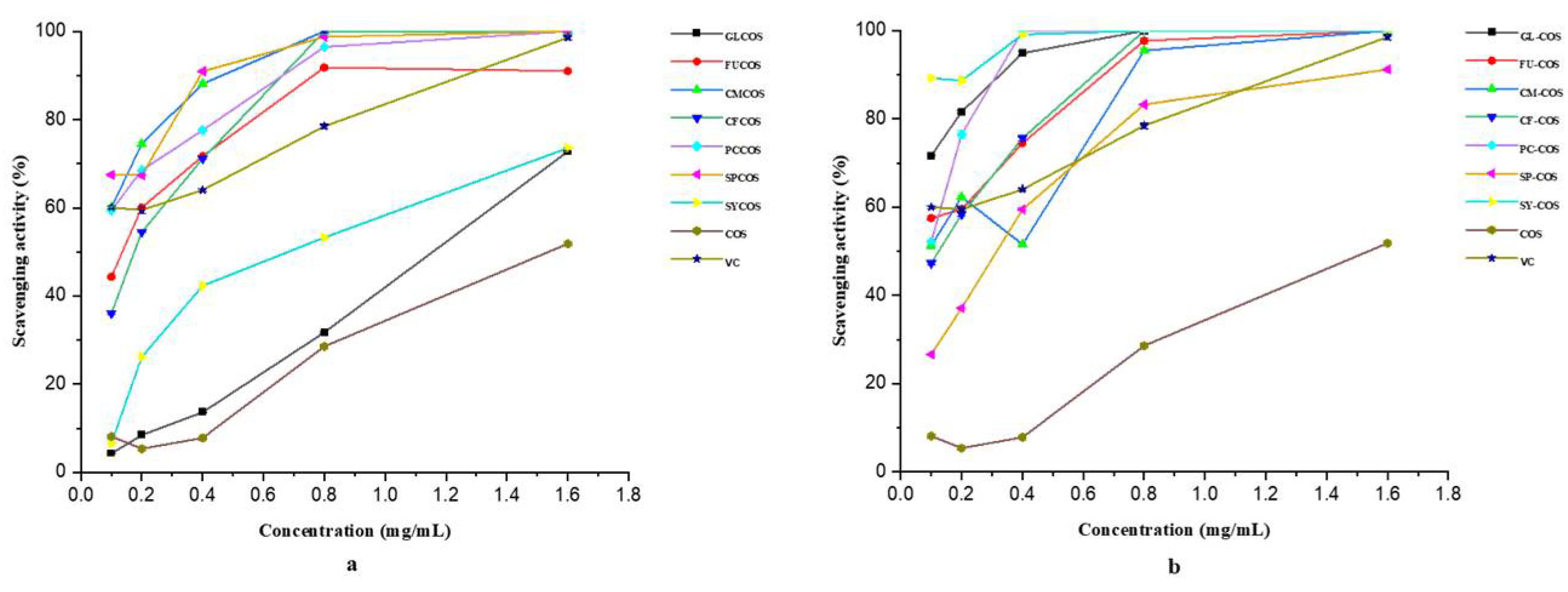
3.2.3. Scavenging Ability against Hydroxyl Radicals
3.2.4. Reducing Power
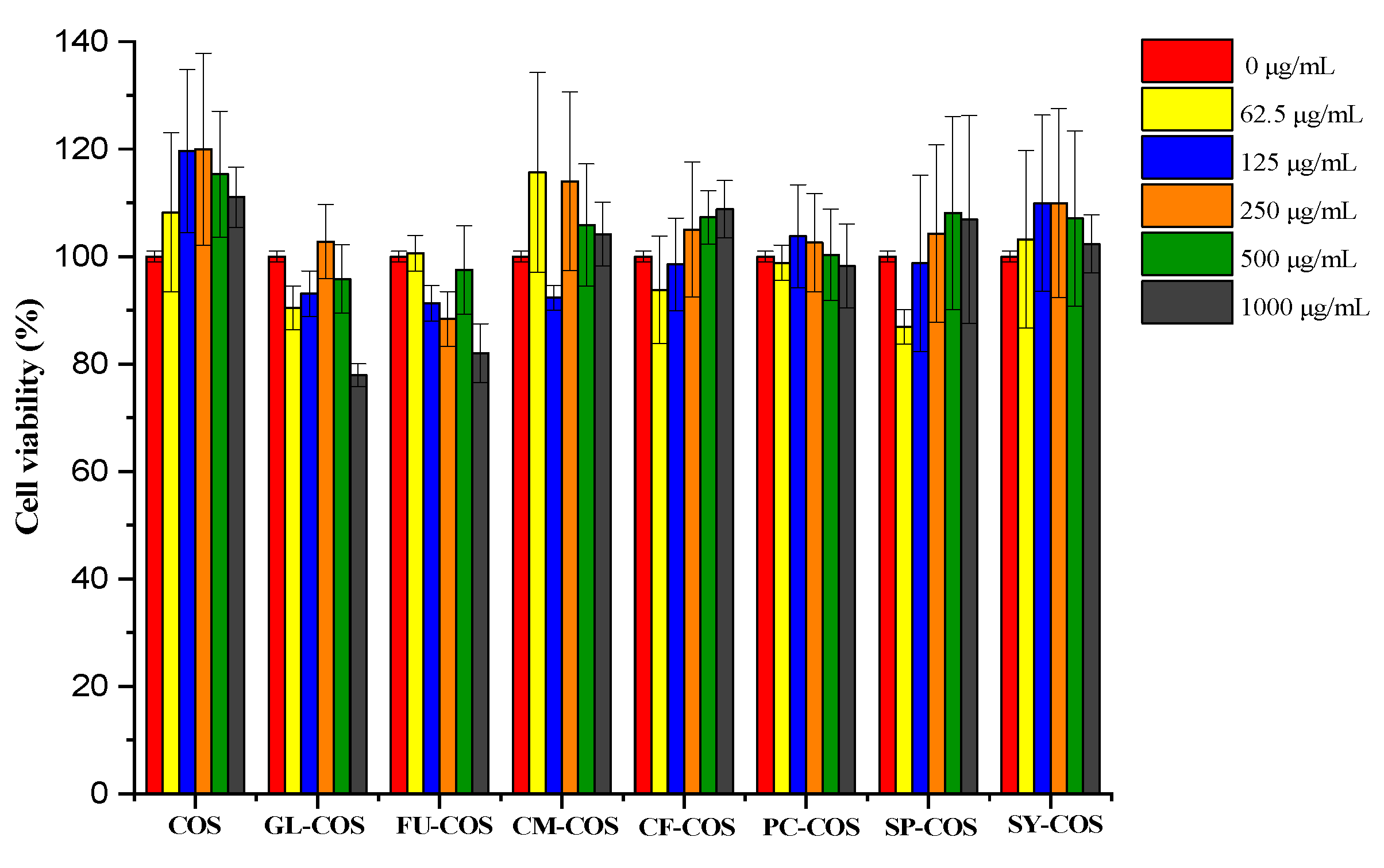
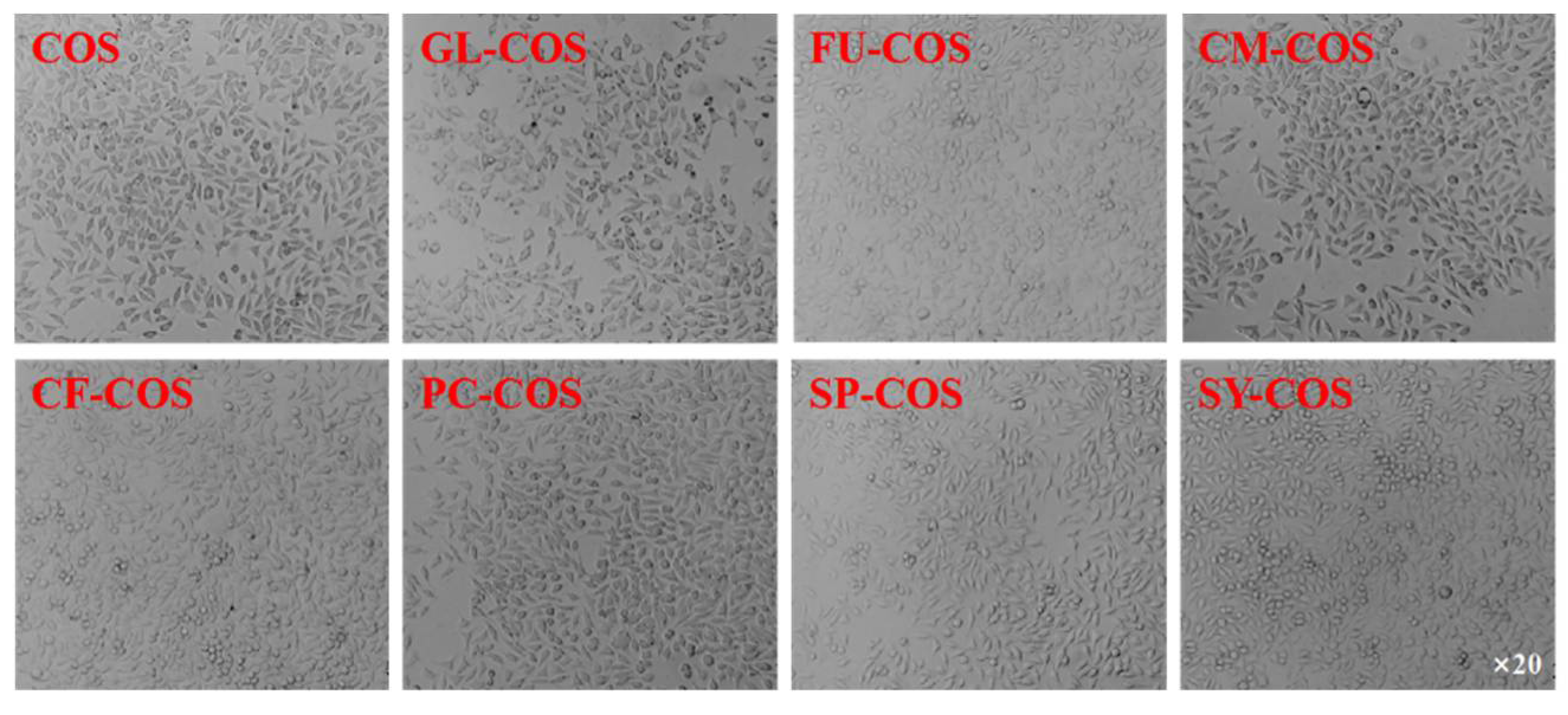
3.3. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. IJCB 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterbourn, C.C. Are free radicals involved in thiol-based redox signaling? Free Radic. Biol. Med. 2015, 80, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Szweda, P.A.; Friguet, B.; Szweda, L.I. Proteolysis, free radicals, and aging. Free Radic. Biol. Med. 2002, 33, 29–36. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Kiokias, S.; Varzakas, T.; Oreopoulou, V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 78–93. [Google Scholar] [CrossRef]
- Cao, G.H.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering-An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, Characterization and Applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Liang, S.; Sun, Y.X.; Dai, X.L. A Review of the Preparation, Analysis and Biological Functions of Chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.E.; Xu, C.J.; Gao, K.; Yang, H.Y.; Liu, Y.L.; Fan, Z.Q.; Liu, S.; Qin, Y.K.; Yu, H.H.; Li, P.C. Characterization of Different Salt Forms of Chitooligosaccharides and Their Effects on Nitric Oxide Secretion by Macrophages. Molecules 2021, 26, 2563. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, J.H.; Song, M.J.; Park, P.J.; Shin, E.S.; Sohn, J.H.; Seo, D.-B.; Lim, K.M.; Kim, W.G.; Lee, S.-J. Effects of Chitooligosaccharide Lactate Salt on Sleep Deprivation-Induced Fatigue in Mice. Biol. Pharm. Bull. 2010, 33, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Mittal, A.; Benjakul, S. Chitosan, Chitooligosaccharides and Their Polyphenol Conjugates: Preparation, Bioactivities, Functionalities and Applications in Food Systems. Food Rev. Int. 2021, 1–23. [Google Scholar] [CrossRef]
- Eom, T.-K.; Senevirathne, M.; Kim, S.-K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharmacol. 2012, 34, 519–527. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Thanh-Sang, V.; Dai-Hung, N.; Long Giang, B.; Dai-Nghiep, N.; Kim, S.-K. The free radical scavenging and anti-inflammatory activities of gallate-chitooligosaccharides in human lung epithelial A549 cells. Process Biochem. 2017, 54, 188–194. [Google Scholar]
- Mittal, A.; Singh, A.; Bin, Z.; Visessanguan, W.; Benjakul, S. Chitooligosaccharide Conjugates Prepared Using Several Phenolic Compounds via Ascorbic Acid/H2O2 Free Radical Grafting: Characteristics, Antioxidant, Antidiabetic, and Antimicrobial Activities. Foods 2022, 11, 920. [Google Scholar] [CrossRef]
- Lee, D.-S.; Woo, J.-Y.; Ahn, C.-B.; Je, J.-Y. Chitosan-hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef]
- Li, Q.; Mi, Y.; Tan, W.; Guo, Z. Highly efficient free radical-scavenging property of phenolic-functionalized chitosan derivatives: Chemicalmodification and activity assessment. Int. J. Biol. Macromol. 2020, 164, 4279–4288. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Tan, W.; Zhang, J.; Guo, Z. Phenolic-containing chitosan quaternary ammonium derivatives and their significantly enhanced antioxidant and antitumor properties. Carbohydr. Res. 2020, 498, 108169. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and characterization of α-lipoic acid grafted chitosan derivatives with antioxidant activity. React. Funct. Polym. 2022, 172, 105205. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Antioxidant and free radical scavenging activities of taxoquinone, a diterpenoid isolated from Metasequoia glyptostroboides. S. Afr. J. Bot. 2017, 111, 93–98. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Mi, Y.; Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of the acetylated chitosan derivatives containing sulfonium salts. Int. J. Biol. Macromol. 2020, 152, 349–358. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between alpha- and beta-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tan, W.; Zhao, P.; Mi, Y.; Guo, Z. Facile synthesis, characterization, antioxidant activity, and antibacterial activity of carboxymethyl inulin salt derivatives. Int. J. Biol. Macromol. 2022, 199, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Kunchandy, E.; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tan, W.; Wei, L.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antioxidant Evaluation of Novel Pyridylurea-Functionalized Chitosan Derivatives. Polymers 2019, 11, 951. [Google Scholar] [CrossRef] [Green Version]
- Benbettaieb, N.; Nyagaya, J.; Seuvre, A.-M.; Debeaufort, F. Antioxidant Activity and Release Kinetics of Caffeic and p-Coumaric Acids from Hydrocolloid-Based Active Films for Healthy Packaged Food. J. Agric. Food Chem. 2018, 66, 6906–6916. [Google Scholar] [CrossRef]
- Mi, Y.; Tan, W.; Zhang, J.; Guo, Z. Modification of Hydroxypropyltrimethyl Ammonium Chitosan with Organic Acid: Synthesis, Characterization, and Antioxidant Activity. Polymers 2020, 12, 2460. [Google Scholar] [CrossRef]
- RiceEvans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wei, L.; Chen, Y.; Mi, Y.; Sun, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis of urea-functionalized chitosan derivatives for potential antifungal and antioxidant applications. Carbohydr. Polym. 2019, 215, 108–118. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Mi, Y.; Miao, Q.; Tan, W.; Li, Q.; Guo, Z. Synthesis, characterization, and antioxidant activity of carboxymethyl chitosan derivatives containing sulfonium salt. J. Oceanol. Limnol. 2022, 40, 284–295. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Ji, X.; Cui, J.; Mi, Y.; Zhang, J.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives. Mar. Drugs 2022, 20, 489. https://doi.org/10.3390/md20080489
Sun Y, Ji X, Cui J, Mi Y, Zhang J, Guo Z. Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives. Marine Drugs. 2022; 20(8):489. https://doi.org/10.3390/md20080489
Chicago/Turabian StyleSun, Yan, Xia Ji, Jingmin Cui, Yingqi Mi, Jingjing Zhang, and Zhanyong Guo. 2022. "Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives" Marine Drugs 20, no. 8: 489. https://doi.org/10.3390/md20080489





