Cyanotoxin Occurrence and Diversity in 98 Cyanobacterial Blooms from Swedish Lakes and the Baltic Sea
Abstract
:1. Introduction
2. Results and Discussion
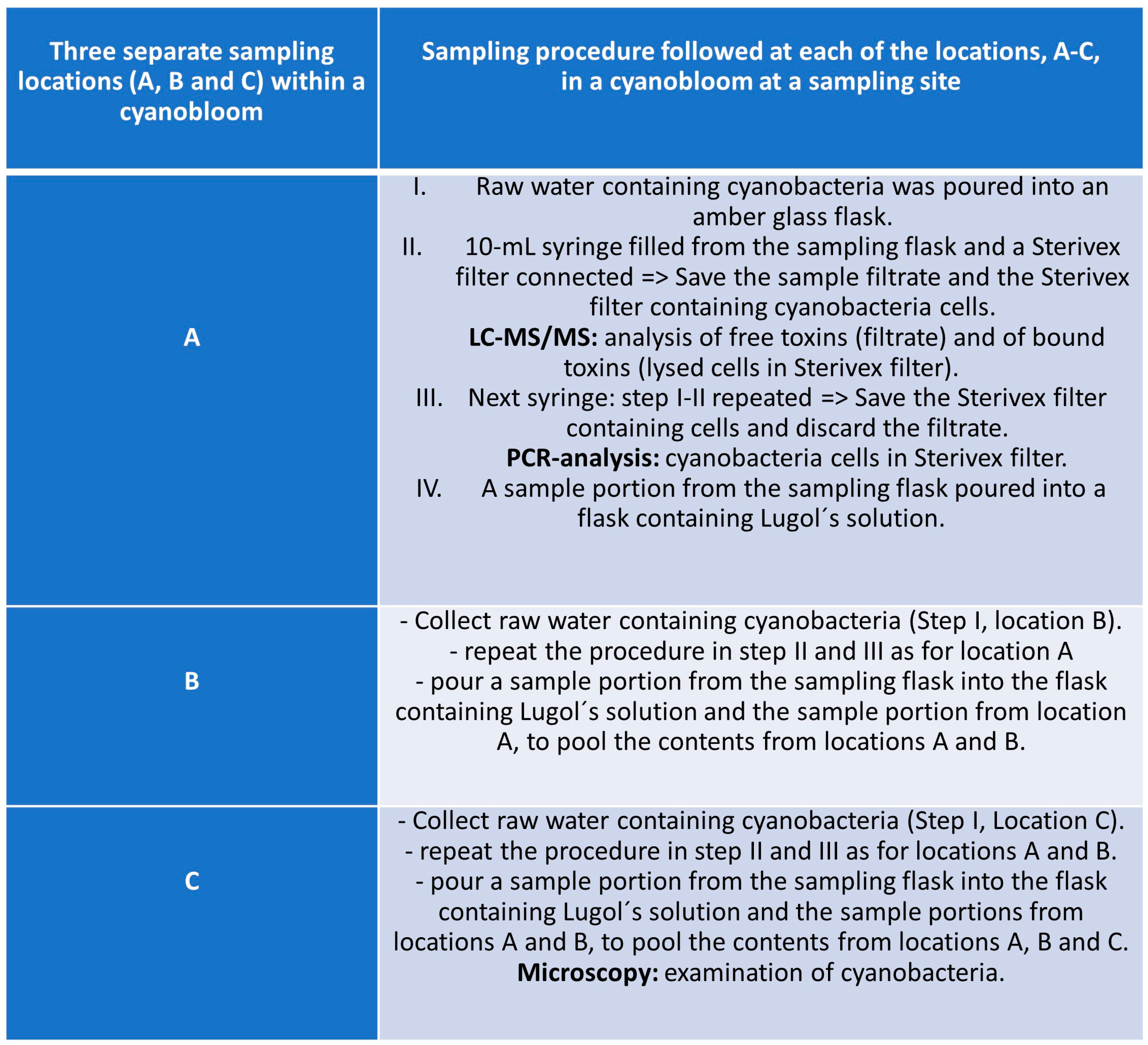
2.1. Study Site Selection and Sampling Strategy
2.2. Diversity of Cyanotoxin Congeners
2.3. Cyanotoxin Quantities
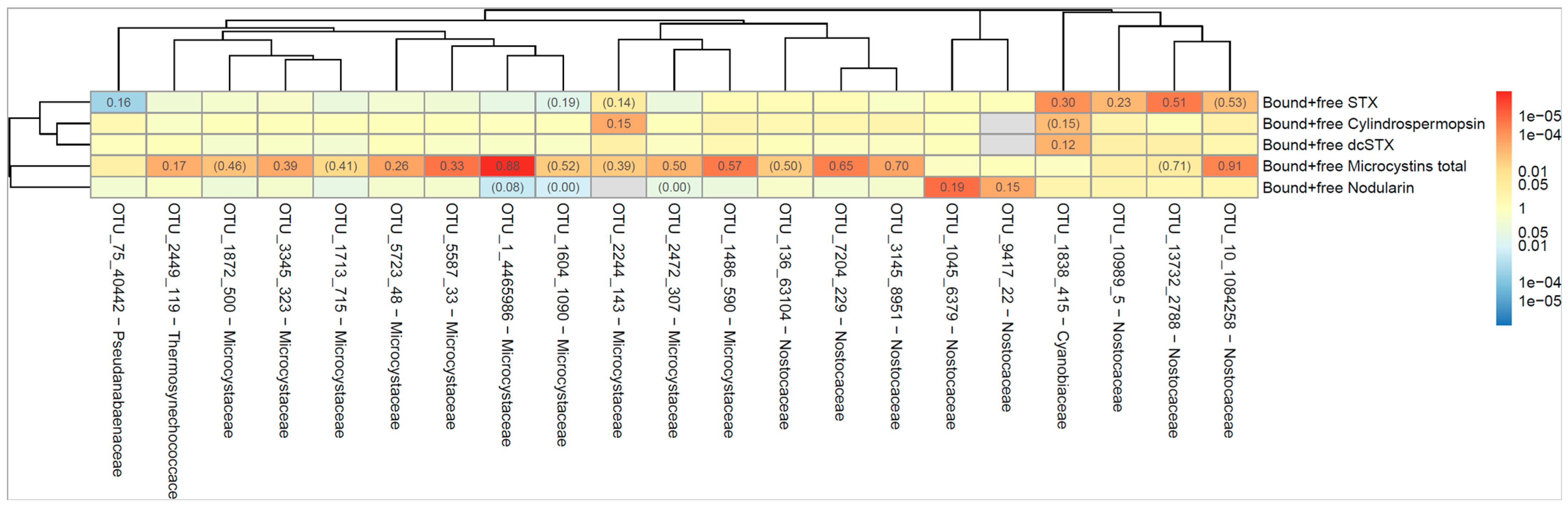
2.4. Molecular Analysis
2.4.1. Total Community Composition of the Samples
2.4.2. Cyanobacterial Community Composition
2.5. Morphological Analysis of Cyanobacteria
3. Materials and Methods
3.1. Chemicals and Reference Standards
3.2. Materials
3.3. Sampling and Sample Preparation
3.3.1. Free Toxins
3.3.2. Cell-Bound Toxins
3.4. Analysis of Cyanotoxins with LC-MS/MS
3.4.1. LC-MS/MS Analysis with C18 Column
3.4.2. LC-MS/MS with HILIC Column
3.5. DNA Extraction, Purification, and 16S rRNA Amplicon Sequencing
3.6. Sequence Analysis
3.7. Microscopy
3.8. Statistical Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manubolu, M.; Eklund, S.; Dutta, P.C.; Malmlöf, K. Variable Exposure and Responses to Cyanotoxins in Cattle Grazing on Pastures in the Coastal Zone of the Baltic Sea: A field Study. Int. J. Environ. Res. 2014, 8, 733–740. [Google Scholar]
- Pekar, H.; Westerberg, E.; Bruno, O.; Lääne, A.; Persson, K.M.; Sundström, L.F.; Thim, A.M. Fast, rugged and sensitive ultra high pressure liquid chromatography tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water--First findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. A 2016, 1429, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kahru, M.; Elmgren, R. Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences 2014, 11, 3619–3633. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef]
- Moreira, C.; Vasconcelos, V.; Antunes, A. Cyanobacterial Blooms: Current Knowledge and New Perspectives. Earth 2022, 3, 127–135. [Google Scholar] [CrossRef]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bashir, F.; Bashir, A.; Bouaïcha, N.; Chen, L.; Codd, G.A.; Neilan, B.; Xu, W.L.; Ziko, L.; Rajput, V.D.; Minkina, T.; et al. Cyanotoxins, biosynthetic gene clusters, and factors modulating cyanotoxin biosynthesis. World J. Microbiol. Biotechnol. 2023, 39, 241. [Google Scholar] [CrossRef]
- Moreira, C.; Gomes, C.; Vasconcelos, V.; Antunes, A. Cyanotoxins Occurrence in Portugal: A New Report on Their Recent Multiplication. Toxins 2020, 12, 154. [Google Scholar] [CrossRef]
- Bloch, R.A.; Faulkner, G.; Hilborn, E.D.; Wismer, T.; Martin, N.; Rhea, S. Geographic Variability, Seasonality, and Increase in ASPCA Animal Poison Control Center Harmful Blue-Green Algae Calls-United States and Canada, 2010–2022. Toxins 2023, 15, 505. [Google Scholar] [CrossRef]
- Turner, A.D.; Turner, F.R.I.; White, M.; Hartnell, D.; Crompton, C.G.; Bates, N.; Egginton, J.; Branscombe, L.; Lewis, A.M.; Maskrey, B.H. Confirmation Using Triple Quadrupole and High-Resolution Mass Spectrometry of a Fatal Canine Neurotoxicosis following Exposure to Anatoxins at an Inland Reservoir. Toxins 2022, 14, 804. [Google Scholar] [CrossRef]
- Menezes, C.; Nova, R.; Vale, M.; Azevedo, J.; Vasconcelos, V.; Pinto, C. First description of an outbreak of cattle intoxication by cyanobacteria (blue-green algae) in the South of Portugal. Bov. Pract. 2019, 53, 66–70. [Google Scholar] [CrossRef]
- Wood, R. Acute animal and human poisonings from cyanotoxin exposure—A review of the literature. Environ. Int. 2016, 91, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As We Drink and Breathe: Adverse Health Effects of Microcystins and Other Harmful Algal Bloom Toxins in the Liver, Gut, Lungs and Beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.Y.; Zhu, X.; Nagata, M.; Loo, L.; Chan, O.; Wong, L.L. Cyanotoxin exposure and hepatocellular carcinoma. Toxicology 2023, 487, 153470. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.; Gadi, S.; Qi, Q.; Rios-Colon, L.; Khatiwada, S.; Vandana; Fernando, R.A.; Levine, K.E.; Kumar, D. Cyanotoxins Increase Cytotoxicity and Promote Nonalcoholic Fatty Liver Disease Progression by Enhancing Cell Steatosis. Toxins 2023, 15, 411. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Tischbein, M.; Cox, P.A.; Stommel, E.W. Cyanotoxins and the Nervous System. Toxins 2021, 13, 660. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef]
- Nugumanova, G.; Ponomarev, E.D.; Askarova, S.; Fasler-Kan, E.; Barteneva, N.S. Freshwater Cyanobacterial Toxins, Cyanopeptides and Neurodegenerative Diseases. Toxins 2023, 15, 233. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto António, D.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; EUR 28624; European Commission: Brussels, Belgium, 2017. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Parulekar, N.N.; Kolekar, P.; Jenkins, A.; Kleiven, S.; Utkilen, H.; Johansen, A.; Sawant, S.; Kulkarni-Kale, U.; Kale, M.; Sæbø, M. Characterization of bacterial community associated with phytoplankton bloom in a eutrophic lake in South Norway using 16S rRNA gene amplicon sequence analysis. PLoS ONE 2017, 12, e0173408. [Google Scholar] [CrossRef]
- Gobler, C.J.; Jankowiak, J.G. Dynamic Responses of Endosymbiotic Microbial Communities Within Microcystis Colonies in North American Lakes to Altered Nitrogen, Phosphorus, and Temperature Levels. Front. Microbiol. 2021, 12, 781500. [Google Scholar] [CrossRef]
- Yang, T.; Lee, C.S.; Cho, J.Y.; Bae, M.J.; Kim, E.J. Comparison of Bacterial Assemblages Associated with Harmful Cyanobacteria under Different Light Conditions. Microorganisms 2022, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Bell, S.G.; Codd, G.A. Production of novel polyclonal antibodies against the cyanobacterial toxin microcystin-LR and their application for the detection and quantification of microcystins and nodularin. Water Res. 2000, 34, 2761–2769. [Google Scholar] [CrossRef]
- Zeck, A.; Eikenberg, A.; Weller, M.G.; Niessner, R. Highly sensitive immunoassay based on a monoclonal antibody specific for [4-arginine]microcystins. Anal. Chim. Acta 2001, 441, 1–13. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Beattie, K.A.; Pflugmacher, S.; Codd, G.A. Immuno-crossreactivity and toxicity assessment of conjugation products of the cyanobacterial toxin, microcystin-LR. FEMS Microbiol. Lett. 2000, 189, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Fewer, D.P.; Neilan, B.A. Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 2013, 37, 23–43. [Google Scholar] [CrossRef]
- Aparicio-Muriana, M.D.M.; Lara, F.J.; Olmo-Iruela, M.D.; García-Campaña, A.M. Determination of Multiclass Cyanotoxins in Blue-Green Algae (BGA) Dietary Supplements Using Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2023, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Klijnstra, M.D.; Faassen, E.J.; Gerssen, A. A Generic LC-HRMS Screening Method for Marine and Freshwater Phycotoxins in Fish, Shellfish, Water, and Supplements. Toxins 2021, 13, 823. [Google Scholar] [CrossRef]
- Jacinavicius, F.R.; Valverde Campos, T.G.; Passos, L.S.; Pinto, E.; Geraldes, V. A rapid LC-MS/MS method for multi-class identification and quantification of cyanotoxins. Toxicon 2023, 234, 107282. [Google Scholar] [CrossRef]
- Sundaravadivelu, D.; Sanan, T.T.; Venkatapathy, R.; Mash, H.; Tettenhorst, D.; DAnglada, L.; Frey, S.; Tatters, A.O.; Lazorchak, J. Determination of Cyanotoxins and Prymnesins in Water, Fish Tissue, and Other Matrices: A Review. Toxins 2022, 14, 213. [Google Scholar] [CrossRef]
- Haddad, S.P.; Bobbitt, J.M.; Taylor, R.B.; Lovin, L.M.; Conkle, J.L.; Chambliss, C.K.; Brooks, B.W. Determination of microcystins, nodularin, anatoxin-a, cylindrospermopsin, and saxitoxin in water and fish tissue using isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2019, 1599, 66–74. [Google Scholar] [CrossRef]
- Turner, A.D.; Waack, J.; Lewis, A.; Edwards, C.; Lawton, L. Development and single-laboratory validation of a UHPLC-MS/MS method for quantitation of microcystins and nodularin in natural water, cyanobacteria, shellfish and algal supplement tablet powders. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1074–1075, 111–123. [Google Scholar] [CrossRef]
- España Amórtegui, J.C.; Pekar, H.; Retrato, M.D.C.; Persson, M.; Karlson, B.; Bergquist, J.; Zuberovic-Muratovic, A. LC-MS/MS Analysis of Cyanotoxins in Bivalve Mollusks-Method Development, Validation and First Evidence of Occurrence of Nodularin in Mussels (Mytilus edulis) and Oysters (Magallana gigas) from the West Coast of Sweden. Toxins 2023, 15, 329. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Masquelier, J.; Andjelkovic, M.; Rajkovic, A. Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products. Separations 2022, 9, 319. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Ahn, A.-C.; Huybrechts, B.; Masquelier, J.; Wilmotte, A.; Andjelkovic, M. LC-MS/MS Validation and Quantification of Cyanotoxins in Algal Food Supplements from the Belgium Market and Their Molecular Origins. Toxins 2022, 14, 513. [Google Scholar] [CrossRef]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Martijn, J.; Lind, A.E.; Schön, M.E.; Spiertz, I.; Juzokaite, L.; Bunikis, I.; Pettersson, O.V.; Ettema, T.J.G. Confident phylogenetic identification of uncultured prokaryotes through long read amplicon sequencing of the 16S-ITS-23S rRNA operon. Environ. Microbiol. 2019, 21, 2485–2498. [Google Scholar] [CrossRef]
- Olrik, K.; Blomqvist, P.; Brettum, P.; Cronberg, G.; Eloranta, P. Methods for Quantitative Assessment of Phytoplankton in Freshwaters. Part I: Sampling, Processing, and Application in Freshwater Environmental Monitoring Programmes; Naturvårdsverket: Stockholm, Sweden, 1998; ISBN 91-620-4860-0. [Google Scholar]
- Edler, L.; Willén, E.; Willén, T.; Ahlgren, G.; Naturvårdsverket, S. Skadliga alger i sjöar och hav; Naturvårdsverket Förlag: Stockholm, Sweden, 1995; ISBN 9789162044473. [Google Scholar]
- Wang, H.; Zhang, Z.; Liang, D.; Du, H.; Pang, Y.; Hu, K.; Wang, J. Separation of wind’s influence on harmful cyanobacterial blooms. Water Res. 2016, 98, 280–292. [Google Scholar] [CrossRef]
- Vander Woude, A.; Ruberg, S.; Johengen, T.; Miller, R.; Stuart, D. Spatial and temporal scales of variability of cyanobacteria harmful algal blooms from NOAA GLERL airborne hyperspectral imagery. J. Great Lakes Res. 2019, 45, 536–546. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Willby, N.J.; Gilvear, D.J. The spatial dynamics of vertical migration by Microcystis aeruginosa in a eutrophic shallow lake: A case study using high spatial resolution time-series airborne remote sensing. Limnol. Oceanogr. 2008, 53, 2391–2406. [Google Scholar] [CrossRef]
- Christoffersen, K.; Lyck, S.; Winding, A. Microbial activity and bacterial community structure during degradation of microcystins. Aquat. Microb. Ecol. 2002, 27, 125–136. [Google Scholar] [CrossRef]
- Holst, T.; Jørgensen, N.O.G.; Jørgensen, C.; Johansen, A. Degradation of microcystin in sediments at oxic and anoxic, denitrifying conditions. Water Res. 2003, 37, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, T.; Ki, J.S. Impact of environmental factors on the regulation of cyanotoxin production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Jiang, X. Cyanobacterial Toxins in Freshwater and Food: Important Sources of Exposure to Humans. Annu. Rev. Food Sci. Technol. 2017, 8, 281–304. [Google Scholar] [CrossRef]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Microbiol. 2012, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, J.G.; Gobler, C.J. The Composition and Function of Microbiomes Within Microcystis Colonies Are Significantly Different Than Native Bacterial Assemblages in Two North American Lakes. Front. Microbiol. 2020, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, Z.; Min, S.; Wang, J.; Min, J. Characteristics of microbial community structure and influencing factors of Yangcheng Lake and rivers entering Yangcheng Lake during the wet season. Environ. Sci. Pollut. Res. Int. 2024, 31, 9565–9581. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. A framework for human microbiome research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Acinas, S.G.; Sarma-Rupavtarm, R.; Klepac-Ceraj, V.; Polz, M.F. PCR-induced sequence artifacts and bias: Insights from comparison of two 16S rRNA clone libraries constructed from the same sample. Appl. Env. Microbiol. 2005, 71, 8966–8969. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Lanzén, A.; Jørgensen, S.L.; Huson, D.H.; Gorfer, M.; Grindhaug, S.H.; Jonassen, I.; Øvreås, L.; Urich, T. CREST—Classification resources for environmental sequence tags. PLoS ONE 2012, 7, e49334. [Google Scholar] [CrossRef]
- WHO. World Health Organization Repport, 2017. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- European Union (EU). Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption; Off J Eur Union. 2020, L435; EU: Brussels, Belgium.
- Livsmedelsverket. Handbok Dricksvattenrisker Cyanotoxiner i Driksvatten; Livsmedelsverket; Uppsala, Sweden; 2018; ISSN 1104-7089. [Google Scholar]

| Toxin | Share of Positive Samples, Only Free Toxins (%) | Share of Positive Samples (%) | Average Free Toxins (µg/L) | SD Free Toxins (µg/L) | Average Total Quantity (µg/L) | SD Total Quantity (µg/L) |
|---|---|---|---|---|---|---|
| Microcystin RR | 19 | 70 | 4.9 | 6.4 | 262.1 | 1782.7 |
| Microcystin LR | 10 | 55 | 148.5 | 132.1 | 197.3 | 682.5 |
| Microcystin WR | 1 | 19 | 0.06 | 0.01 | 6.5 | 9.7 |
| Microcystin LA | 1 | 4 | 0.2 | 0.02 | 37.1 | 14.4 |
| Microcystin LY | 3 | 11 | 2.8 | 0.8 | 1.7 | 1.4 |
| Microcystin LW | 1 | 8 | 0.6 | 0.06 | 1.5 | 0.7 |
| Microcystin LF | 2 | 8 | 2.5 | 0.5 | 1.2 | 0.8 |
| Microcystin HtyR | 1 | 13 | 0 | 0.01 | 0.3 | 0.2 |
| Microcystin HilR | 2 | 16 | 3.4 | 0.6 | 6.9 | 7.9 |
| Microcystin HtyR [D-Asp3, (E)-Dhb7] | 2 | 9 | 2.8 | 0.5 | 4.9 | 3.2 |
| Microcystin RR [Dha 7] | 20 | 46 | 0.7 | 0.8 | 6.0 | 9.6 |
| Microcystin YR | 11 | 36 | 0.9 | 0.3 | 38.1 | 110.0 |
| Microcystin RR [D-Asp3] + Microcystin RR [D-Asp3, (E)-Dhb7] | 10 | 39 | 0.5 | 0.3 | 5.2 | 8.5 |
| Microcystin LR [D-Asp3] | 3 | 22 | 0.4 | 0.08 | 14. | 17.7 |
| Microcystin (N-methyl-L) R | 4 | 17 | 1.3 | 0.4 | 5.8 | 6.9 |
| Microcystin HphR [D-Asp3, (E)-Dhb7] | 1 | 10 | 1.0 | 0.1 | 138.7 | 135.6 |
| Microcystins * | 39 | 82 | 42.0 | 139.1 | 406.9 | 2619.9 |
| Micropeptin 1106 | 0 | 1 | 0 | 0 | 0.1 | 0.01 |
| Anatoxin-a | 1 | 4 | 1.8 | 0.2 | 2.6 | 0.6 |
| Homoanatoxin-a | 4 | 6 | 0.2 | 0.03 | 0.2 | 0.04 |
| Cylindrosper- mopsin | 9 | 10 | 1.1 | 0.8 | 1.1 | 0.9 |
| Nodularin | 8 | 13 | 21.7 | 13.6 | 155.5 | 152.7 |
| Decarbamoyl- saxitoxin | 7 | 9 | 1.6 | 1.5 | 4.6 | 1.9 |
| Saxitoxin | 36 | 45 | 19.1 | 188.3 | 72.9 | 188.9 |
| Decarbamoyl- neosaxitoxin | 0 | 0 | 0 | 0 | 0 | 0 |
| Saxitoxins * | 36 | 47 | 19.3 | 189.7 | 72.0 | 190.4 |
| Taxonomic Affiliation | OTU Number | Number of Samples with Presence of Each OTU |
|---|---|---|
| Nostocales; Nostocaceae; Aphanizomenon NIES 81 | OTU_10 | 243 |
| Synechococcales; Cyanobiaceae; Cyanobium PCC 6307 | OTU_13563 | 222 |
| Nostocales; Microcystaceae; Microcystis PCC 7914 | OTU_1 | 215 |
| Synechococcales; Cyanobiaceae; Cyanobium PCC 6307 | OTU_17512 | 202 |
| Synechococcales; Cyanobiaceae; Cyanobium PCC 6307 | OTU_14559 | 200 |
| Nostocales; Nostocaceae | OTU_933 | 191 |
| Synechococcales; Cyanobiaceae; Cyanobium PCC 6307 | OTU_883 | 187 |
| Nostocales; Microcystaceae; Snowella 0TU37S04 | OTU_9 | 178 |
| Nostocales; Nostocaceae; Aphanizomenon NIES 81 | OTU_13732 | 156 |
| Nostocales; Microcystaceae; Snowella 0TU37S04 | OTU_250 | 147 |
| Year | Month | Number of Sampling Sites |
|---|---|---|
| 2016 | July | 1 |
| August | 10 | |
| September | 10 | |
| October | 3 | |
| 2017 | June | 1 |
| July | 16 | |
| August | 44 | |
| September | 12 | |
| October | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirks, C.; Cappelli, P.; Blomqvist, M.; Ekroth, S.; Johansson, M.; Persson, M.; Drakare, S.; Pekar, H.; Zuberovic Muratovic, A. Cyanotoxin Occurrence and Diversity in 98 Cyanobacterial Blooms from Swedish Lakes and the Baltic Sea. Mar. Drugs 2024, 22, 199. https://doi.org/10.3390/md22050199
Dirks C, Cappelli P, Blomqvist M, Ekroth S, Johansson M, Persson M, Drakare S, Pekar H, Zuberovic Muratovic A. Cyanotoxin Occurrence and Diversity in 98 Cyanobacterial Blooms from Swedish Lakes and the Baltic Sea. Marine Drugs. 2024; 22(5):199. https://doi.org/10.3390/md22050199
Chicago/Turabian StyleDirks, Caroline, Paolo Cappelli, Maria Blomqvist, Susanne Ekroth, Malin Johansson, Max Persson, Stina Drakare, Heidi Pekar, and Aida Zuberovic Muratovic. 2024. "Cyanotoxin Occurrence and Diversity in 98 Cyanobacterial Blooms from Swedish Lakes and the Baltic Sea" Marine Drugs 22, no. 5: 199. https://doi.org/10.3390/md22050199





