Sphaeroane and Neodolabellane Diterpenes from the Red Alga Sphaerococcus coronopifolius
Abstract
:1. Introduction
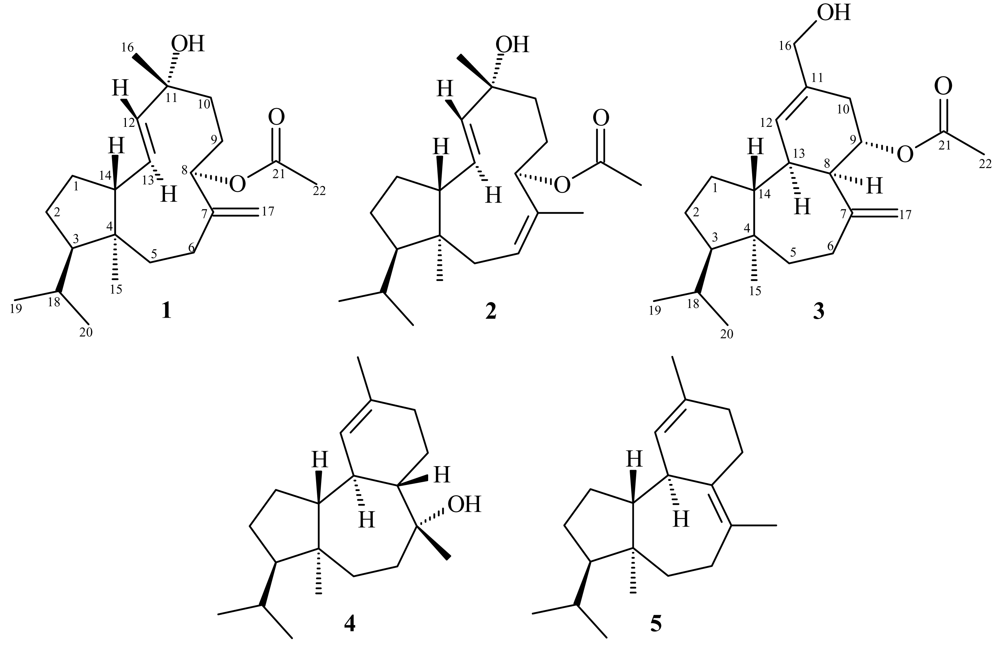
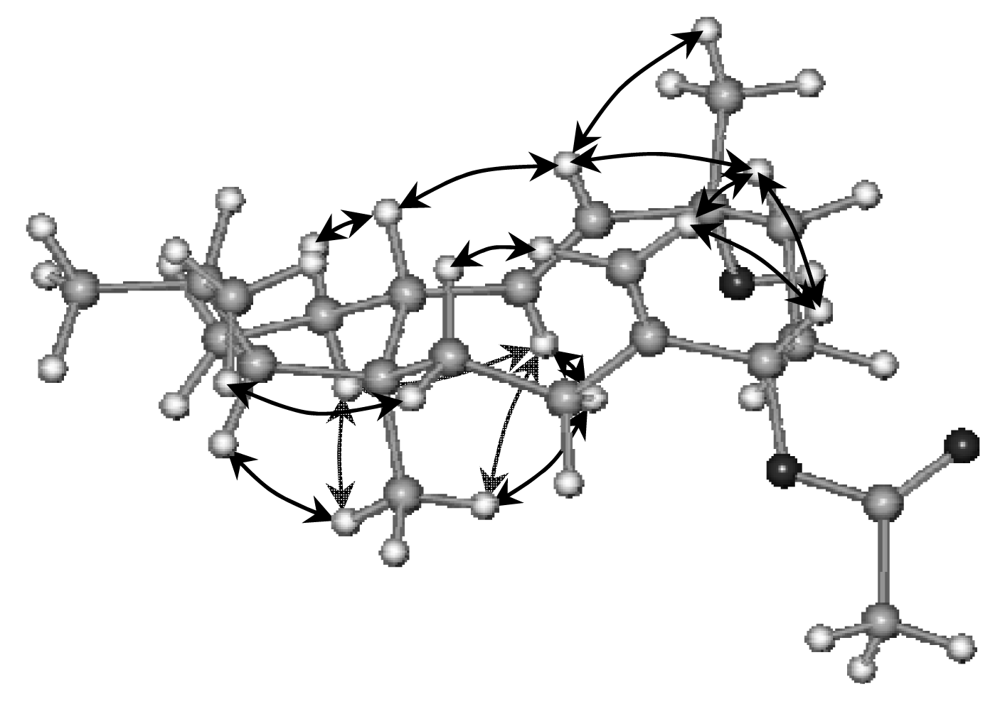
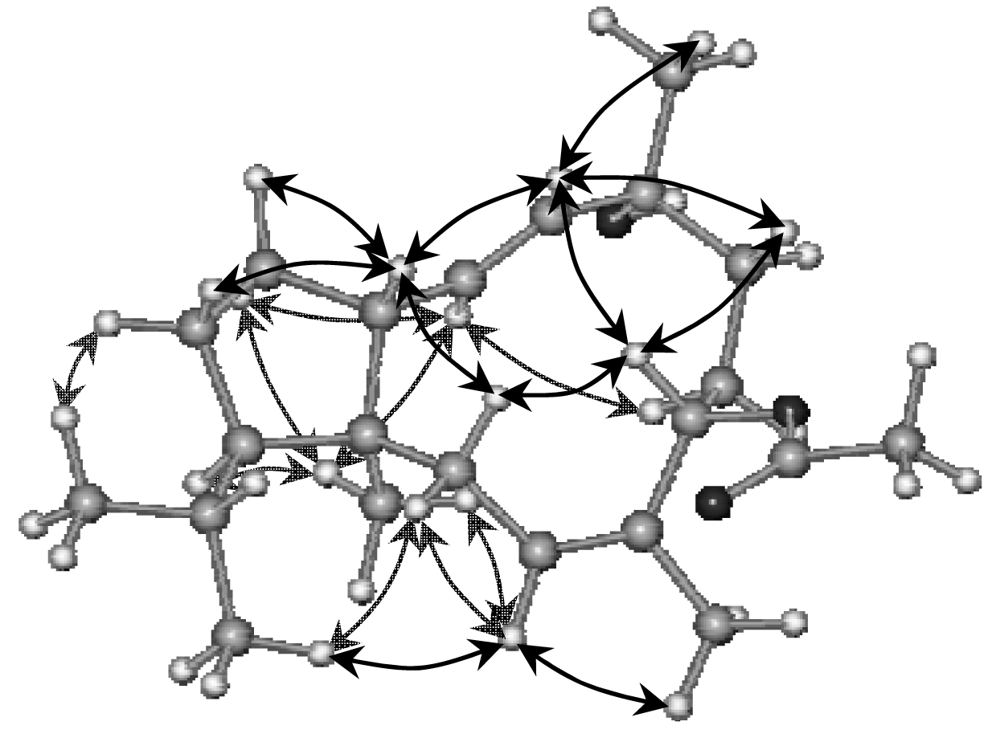
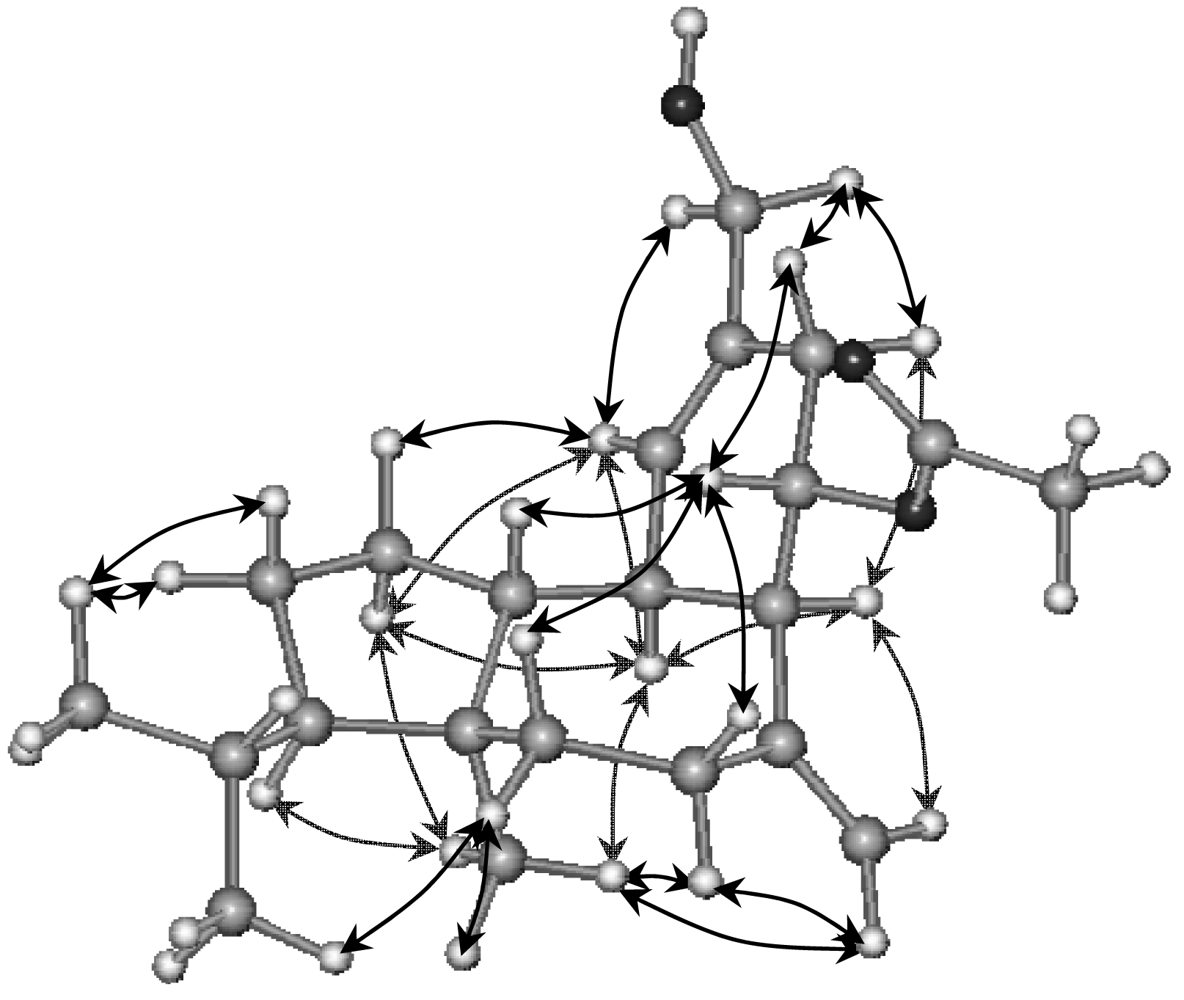
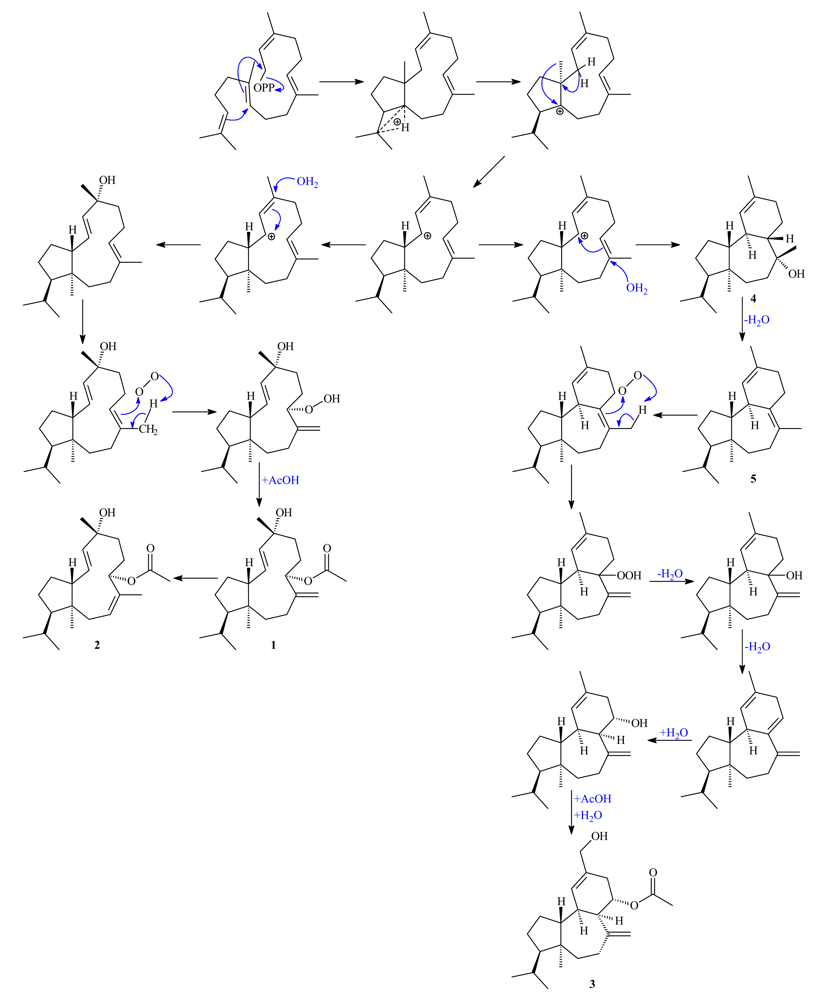
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
Sphaerollane-I (1)
Sphaerollane-II (2)
16-Hydroxy-9S*-acetoxy-8-epi-sphaerodiene-2 (3)
Acknowledgements
- Samples Availability: Available from the authors.
References
- Gershenzon, J; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat Chem Biol 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V; Vagias, C; Rahman, MM; Gibbons, S; Roussis, V. Brominated Diterpenes with Antibacterial Activity from the Red Alga Sphaerococcus coronopifolius. J Nat Prod 2008, 71, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V; Quesada, A; Vagias, C; Moreau, D; Roussakis, C; Roussis, V. Cytotoxic Bromoditerpenes from the Red Alga Sphaerococcus coronopifolius. Tetrahedron 2008, 64, 5184–5190, and references cited therein. [Google Scholar] [CrossRef]
- Kladi, M; Vagias, C; Stavri, M; Rahman, MM; Gibbons, S; Roussis, V. C15 Acetogenins with Antistaphylococcal Activity from the Red Alga Laurencia glandulifera. Phytochem Lett 2008, 1, 31–36. [Google Scholar] [CrossRef]
- Kontiza, I; Stavri, M; Zloh, M; Vagias, C; Gibbons, S; Roussis, V. New Metabolites with Antibacterial Activity from the Marine Angiosperm Cymodocea nodosa. Tetrahedron 2008, 64, 1696–1702. [Google Scholar] [CrossRef]
- Cafieri, F; de Napoli, L; Fattorusso, E; Piattelli, M; Sciuto, S. Presphaerol, a New Rearranged Diterpene from the Alga Sphaerococcus coronopifolius. Tetrahedron Lett 1979, 20, 963–966. [Google Scholar] [CrossRef]
- Cafieri, F; Fattorusso, E; di Blasio, B; Redone, C. Diterpenes from the Red Alga Sphaerococcus coronopifolius. Structure of Sphaerodiene and Reassignment of Sstructure for Presphaerol. Tetrahedron Lett 1981, 22, 4123–4126. [Google Scholar] [CrossRef]
- Lee, J; Hong, J. First Synthesis and Structural Elucidation of (−)-Presphaerene. J Org Chem 2004, 69, 6433–6440. [Google Scholar] [CrossRef] [PubMed]
- Cafieri, F; de Napoli, L; Fattorusso, E; Santacroce, C. Diterpenes from the Red Alga Sphaerococcus coronopifolius. Phytochemistry 1987, 26, 471–473. [Google Scholar] [CrossRef]
- Cafieri, F; Fattorusso, E; Santacroce, C. Bromocorodienol, a Diterpenoid Based on a Novel Bicyclic Skeleton from the Red Alga Sphaerococcus coronopifolius. Tetrahedron Lett 1984, 25, 3141–3144. [Google Scholar] [CrossRef]
- Bowden, BF; Cusack, BJ; Dangel, A. 13-Epi-9-deacetoxyxenicin, a Cytotoxic Diterpene from the Soft Coral Asterospicularia laurae (Alcyonacea). Mar Drugs 2003, 1, 18–26. [Google Scholar]
- Bowden, BF; Coll, JC; Gulbis, JM; Mackay, MF; Willis, RH. Studies of Australian Soft Corals. XXXVIII. Structure Determination of Several Diterpenes Derived from a Cespitularia sp. (Coelenterata, Octocorallia, Xeniidae). Aust J Chem 1986, 39, 803–812. [Google Scholar] [CrossRef]
- Bowden, BF; Braekman, J; Mitchell, SJ. Studies of Australian Soft Corals. XX. A New Sesquiterpene Furan from Soft Corals of the Family Xeniidae and an Examination of Clavularia inflata from North Queensland Waters. Aust J Chem 1980, 33, 927–932. [Google Scholar] [CrossRef]
- Bowden, BF; Coll, JC; Mitchell, SJ; Stokie, GJ; Blount, JF. Studies of Australian Soft Corals. VIII. A Chemical and Crystallographic Study of a Novel Bicyclic Diterpene Alcohol with a Rearranged Skeleton from an Unknown Species of Soft Coral. Aust J Chem 1978, 31, 2039–2047. [Google Scholar] [CrossRef]
- Kobayashi, M; Son, BW; Fujiwara, T; Kyogoku, Y; Kitagawa, I. Neodolabelline, a Methyl Migrated Dolabellane-type Diterpene from the Okinawan Soft Coral Clavularia koellikeri. Tetrahedron Lett 1984, 25, 5543–5546. [Google Scholar] [CrossRef]
- Subrahmanyam, C; Venkateswara Rao, C; Anjaneyulu, V; Satyanarayana, P; Subba Rao, PV; Ward, RS; Pelter, A. New Diterpenes from a New Species of Lobophytum Soft Coral of the South Andaman Coast. Tetrahedron 1992, 48, 3111–3120. [Google Scholar] [CrossRef]
- Engler, M; Anke, T; Sterner, O. Tintinnadiol, a Sphaeroane Diterpene from Fruiting Bodies of Mycena tintinnabulum. Phytochemistry 1998. [Google Scholar]
| 1 | 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pos. | δH | mult, J | NOESY | δCb | HMBC (C→H) | δH | mult, J | NOESY | δCb | HMBC (C→H) |
| 1 | β 1.47
α 1.36 | m
m | 14
13, 15 | 27.9 t | 2, 3 | β 1.57
α 1.41 | m
m | 14
13, 15 | 28.2 t | 2β, 3 |
| 2 | a 1.85
b 1.81 | m
m | 27.1 t | 1α, 1β, 18 | α 1.87
β 1.30 | m
m | 20
14 | 28.1 t | 3 | |
| 3 | 1.21 | m | 15 | 55.4 d | 1α, 1β, 2a, 15, 18, 19, 20 | 1.29 | m | 15 | 57.0 d | 15, 19, 20 |
| 4 | 47.1 s | 1α, 2a, 2b, 3, 5β, 15 | 47.1 s | 14, 15 | ||||||
| 5 | α 2.07
β 1.28 | m
m | 20
17b | 37.0 t | 3, 15 | β 2.52
α 2.28 | dd 14.9, 12.0
ddq 14.9, 6.2, 1.2 | 8, 14
6, 19 | 36.3 t | 14, 15 |
| 6 | α 2.16
β 1.97 | m
m | 13, 15 | 28.4 t | 5β, 17a, 17b | 5.25 | ddq 12.0, 6.2, 1.2 | 5α, 15, 17, 19 | 127.1 d | 5α, 5β, 8, 17 |
| 7 | 149.8 s | 5β, 8, 17a | 132.8 s | 5α, 17 | ||||||
| 8 | 5.41 | brd 7.9 | 10β, 17a | 75.7 d | 6β, 9b, 10α,10β, 17a, 17b | 5.86 | dd 9.1, 1.6 | 5β, 12, 9α, 10β | 73.8 d | 9α, 17, 10α, 10β |
| 9 | a 1.89
b 1.42 | m
m | 28.3 t | 8, 10α, 10β | β 1.76
α 1.51 | m
m | 8, 13 | 27.5 t | 8, 10α | |
| 10 | α 1.66
β 1.48 | ddd 14.5, 7.9, 1.2
m | 8, 12, 17a | 38.2 t | 8, 9a, 9b, 16 | α 1.63
β 1.49 | m
m | 8, 12 | 38.3 t | 8, 9α, 16 |
| 11 | 73.4 s | 9a, 9b, 10α, 12, 13, 16 | 73.0 s | 12, 13, 16 | ||||||
| 12 | 5.52 | d 15.3 | 10β, 14, 16 | 137.3 d | 10α, 13, 16 | 5.66 | d 15.3 | 8, 10β, 14, 16 | 137.1 d | 10α, 13, 16 |
| 13 | 5.66 | dd 15.3, 10.0 | 1α, 6α, 15 | 127.5 d | 1α, 12 | 5.49 | dd 15.3, 10.4 | 1α, 9α, 15 | 129.1 d | 12 |
| 14 | 2.18 | m | 1β, 12 | 59.4 d | 3, 5β, 15 | 2.22 | m | 1β, 2β, 5β, 12 | 59.7 d | 5β, 12, 15 |
| 15 | 0.77 | s | 1α, 3, 6α, 13 | 10.9 q | 3, 5β | 0.67 | s | 1α, 3, 6, 13 | 12.5 q | 14 |
| 16 | 1.24 | s | 12 | 30.7 q | 10α | 1.29 | s | 12 | 30.3 q | |
| 17 | a 4.74
b 4.65 | brt 1.2
brs | 8, 10β
5β | 107.0 t | 6β, 8 | 1.61 | t 1.2 | 6 | 17.6 q | 6, 8 |
| 18 | 1.56 | brhept
6.6 | 30.3 d | 3, 19, 20 | 1.53 | m | 31.0 d | 19, 20 | ||
| 19 | 0.83 | d 6.6 | 22.5 q | 3, 18, 20 | 0.98 | d 6.6 | 5α, 6 | 22.6 q | 20 | |
| 20 | 0.95 | d 6.6 | 5α | 23.5 q | 3, 18, 19 | 0.85 | d 6.6 | 2α | 22.8 q | 19 |
| 21 | 170.8 s | 8, 22 | 171.3 s | 8, 22 | ||||||
| 22 | 2.06 | s | 21.2 q | 1.99 | s | 21.3 q | ||||
| 3a | 4b | 5b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos. | δH | mult, J | NOESY | δCc | HMBC (C→H) | δH | mult, J | δCc | δH | mult, J | δCc |
| 1 | β 1.72
α 1.25 | m
m | 12
12, 13, 15 | 27.8 t | 14 | a 1.69
b 1.38 | m
m | 27.2 t | a 1.34
b 1.09 | m
m | 26.4 t |
| 2 | a 1.72
b 1.28 | m
m | 19
19 | 26.0 t | 3 | a 1.66
b 1.16 | m
m | 28.2 t | a 1.65
b 1.35 | m
m | 25.9 t |
| 3 | 1.15 | m | 15 | 58.0 d | 1α, 2b, 5β, 15, 19, 20 | 1.06 | m | 59.4 d | 0.95 | m | 58.7 d |
| 4 | 46.4 s | 1β, 2a, 3, 5α, 5β, 6α, 6β, 15 | 45.3 s | 46.4 s | |||||||
| 5 | α 1.87
β 1.48 | ddd 13.7, 7.0, 5.8
m | 15, 20
9 | 38.9 t | 6α, 6β, 14, 15 | a 1.50
b 1.49 | m
m | 37.8 t | a 1.85
b 1.06 | dd 12.8, 7.4
t 12.8 | 40.0 t |
| 6 | α 2.23
β 2.22 | m
m | 15, 17
9 | 30.8 t | 5α, 5β, 17 | a 1.73
b 1.36 | m
m | 37.2 t | a 2.46
b 1.72 | m
m | 30.3 t |
| 7 | 149.4 s | 5α, 5β, 6α, 6β, 17 | 74.4 s | 129.0 s | |||||||
| 8 | 2.74 | dd 11.2, 5.4 | 10α, 13, 17 | 51.3 d | 6α, 6β, 9, 10β, 12, 17 | 1.67 | m | 48.2 d | 135.8 s | ||
| 9 | 5.35 | ddd 11.2, 8.3, 6.2 | 5β, 6β, 10β, 14 | 68.7 d | 8, 10α, 10β | a 1.74
b 1.49 | m
m | 22.5 t | a 1.59
b 1.11 | ddd 13.7,4.2, 3.7
dt 13.7, 7.9 | 26.0 t |
| 10 | β 2.59
α 2.04 | dd 17.0, 6.2
dd 17.0, 8.3 | 9, 16
8, 16 | 32.7 t | 8, 12, 16 | a 1.91
b 1.89 | m
m | 30.8 t | a 1.94
b 1.33 | m
m | 30.2 t |
| 11 | 133.5 s | 10α, 10β, 16 | 132.4 s | 133.9 s | |||||||
| 12 | 5.68 | brdd 2.9, 1.2 | 1α, 1β, 13, 16 | 127.4 d | 10α, 10β, 13, 16 | 5.39 | dm 3.5 | 126.8 d | 5.42 | brs | 124.6
d |
| 13 | 2.28 | m | 1α, 8, 12, 15 | 41.6 d | 1α, 8, 9, 12, 17 | 2.45 | dm 3.5 | 36.1 d | 2.99 | brs | 37.3 d |
| 14 | 1.65 | m | 9 | 53.7 d | 5α, 8, 15 | 1.61 | m | 50.4 d | 1.35 | m | 53.0 d |
| 15 | 0.76 | s | 1α, 3, 5α, 6α, 13, 17 | 13.9 q | 3, 5β | 0.90 | s | 15.1 q | 0.77 | s | 13.0 q |
| 16 | a 3.99
b 3.99 | brs
brs | 10α, 10β, 12 | 66.8 t | 10β, 12 | 1.67 | brs | 23.7 q | 1.73 | brs | 24.1 q |
| 17 | a 4.79
b 4.79 | brs
brs | 6α, 8, 15 | 114.1 t | 6α, 6β, 8 | 1.10 | s | 29.6 q | 1.76 | brs | 20.7 q |
| 18 | 1.53 | m | 29.8 d | 2b, 3, 19, 20 | 1.51 | m | 30.9 d | 1.49 | dhept
9.1, 6.6 | 30.9 d | |
| 19 | 0.82 | d 6.6 | 2a, 2b | 22.4 q | 18, 20 | 0.89 | d 6.7 | 22.9 q | 0.96 | d 6.6 | 23.5 q |
| 20 | 0.92 | d 6.6 | 5α | 23.7 q | 18, 20 | 0.98 | d 6.7 | 23.6 q | 0.86 | d 6.6 | 23.2 q |
| 21 | 170.8 s | 9, 22 | |||||||||
| 22 | 1.99 | s | 21.2 q | ||||||||
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Smyrniotopoulos, V.; Vagias, C.; Roussis, V. Sphaeroane and Neodolabellane Diterpenes from the Red Alga Sphaerococcus coronopifolius. Mar. Drugs 2009, 7, 184-195. https://doi.org/10.3390/md7020184
Smyrniotopoulos V, Vagias C, Roussis V. Sphaeroane and Neodolabellane Diterpenes from the Red Alga Sphaerococcus coronopifolius. Marine Drugs. 2009; 7(2):184-195. https://doi.org/10.3390/md7020184
Chicago/Turabian StyleSmyrniotopoulos, Vangelis, Constantinos Vagias, and Vassilios Roussis. 2009. "Sphaeroane and Neodolabellane Diterpenes from the Red Alga Sphaerococcus coronopifolius" Marine Drugs 7, no. 2: 184-195. https://doi.org/10.3390/md7020184






