Biological Activity of Volatiles from Marine and Terrestrial Bacteria
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
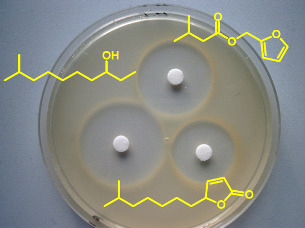
3.1. Antimicrobial assay
3.2. Cell proliferation assay
3.3. Acylhomoserine lactone sensors
Acknowledgements
References
- Bull, AT; Stach, JEM. Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol 2007, 15, 491–499. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244. [Google Scholar]
- Schulz, S; Dickschat, JS. Bacterial volatiles: the smell of small organisms. Nat Prod Rep 2007, 24, 814–842. [Google Scholar]
- Kai, M; Haustein, M; Molina, F; Petri, A; Scholz, B; Piechulla, B. Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 2009, 81, 1001–1012. [Google Scholar]
- Bruce, A; Stewart, D; Verrall, S; Wheatley, RE. Effect of volatiles from bacteria and yeast on the growth and pigmentation of sapstain fungi. Int Biodeterior Biodegradation 2003, 51, 101–108. [Google Scholar]
- Bruce, A; Verrall, S; Hackett, CA; Wheatley, RE. Identification of volatile organic compounds (VOCs) from bacteria and yeast causing growth inhibition of sapstain fungi. Holzforschung 2004, 58, 193–198. [Google Scholar]
- Chaurasia, B; Pandey, A; Palni, LMS; Trivedi, P; Kumar, B; Colvin, N. Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 2005, 160, 75–81. [Google Scholar]
- Vespermann, A; Kai, M; Piechulla, B. Rhizobacterial Volatiles Affect the Growth of Fungi and Arabidopsis thaliana. Appl Environ Microbiol 2007, 73, 5639–5641. [Google Scholar]
- Kai, M; Effmert, U; Berg, G; Piechulla, B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Mikrobiol 2007, 187, 351–360. [Google Scholar]
- Chuankun, X; Minghe, M; Leming, Z; Keqin, Z. Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol Biochem 2004, 36, 1997–2004. [Google Scholar]
- Zou, CS; Mo, MH; Gu, YQ; Zhou, JP; Zhang, KQ. Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol Biochem 2007, 39, 2371–2379. [Google Scholar]
- Fernando, WGD; Ramarathnam, R; Krishnamoorthy, AS; Savchuk, SC. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 2005, 37, 955–964. [Google Scholar]
- Lee, J; Jayaraman, A; Wood, TK. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 2007, 7, 42. [Google Scholar]
- Mueller, RS; Beyhan, S; Saini, SG; Yildiz, FH; Bartlett, DH. Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J Bacteriol 2009, 191, 3504–3516. [Google Scholar]
- Wrigley, DM. Inhibition of Clostridium perfringens sporulation by Bacteroides fragilis and short-chain fatty acids. Anaerobe 2004, 10, 295–300. [Google Scholar]
- Thiel, V; Kunze, B; Verma, P; Wagner-Döbler, I; Schulz, S. New Structural Variants of Homoserine Lactones in Bacteria. Chembiochem 2009, 10, 1861–1868. [Google Scholar]
- Blair, JM; Piddock, LJ. Structure, function and inhibiton of RND efflux in Gram-negative bacteria: an update. Curr Opin Microbiol 2009, 12, 512–519. [Google Scholar]
- Schulz, S; Groenhagen, U; Müller, R. University of Braunschweig—Institute of Technology, Braunschweig, Germany. New pyridine alkaloids in Streptomyces sp. 2010, unpublished work. [Google Scholar]
- Dickschat, JS; Wenzel, SC; Bode, HB; Müller, R; Schulz, S. Biosynthesis of volatiles by the myxobacterium Myxococcus xanthus. Chembiochem 2005, 5, 778–787. [Google Scholar]
- Dickschat, JS; Bode, HB; Wenzel, SC; Müller, R; Schulz, S. Biosynthesis and identification of volatiles released by the myxobacterium Stigmatella aurantiaca. Chembiochem 2005, 6, 2023–2033. [Google Scholar]
- Dickschat, JS; Martens, T; Brinkhoff, T; Simon, M; Schulz, S. Volatiles released by a Streptomyces species isolated from the North Sea. Chem Biodivers 2005, 2, 837–865. [Google Scholar]
- Stritzke, K; Schulz, S; Laatsch, H; Helmke, E; Beil, W. Novel caprolactones from a marine streptomycete. J Nat Prod 2004, 67, 395–401. [Google Scholar]
- Dickschat, JS; Helmke, E; Schulz, S. Volatile organic compounds from arctic bacteria of the Cytophaga-Flavobacterium-Bacteroides group: A retrobiosynthetic approach in chemotaxonomic investigations. Chem Biodivers 2005, 2, 318–353. [Google Scholar]
- Böröczky, K; Laatsch, H; Wagner-Döbler, I; Stritzke, K; Schulz, S. Cluster analysis as selection and dereplication tool for the identification of new natural compounds from large sample sets. Chem Biodivers 2006, 3, 622–634. [Google Scholar]
- Schulz, S; Fuhlendorff, J; Reichenbach, H. Identification and synthesis of volatiles released by the myxobacterium Chondromyces crocatus. Tetrahedron 2004, 60, 3863–3872. [Google Scholar]
- Dickschat, JS; Reichenbach, H; Wagner-Döbler, I; Schulz, S. Novel pyrazines from the myxobacterium Chondromyces crocatus and marine bacteria. Eur J Org Chem 2005, 2005, 4141–4153. [Google Scholar]
- Dickschat, JS; Bode, HB; Kroppenstedt, RM; Müller, R; Schulz, S. Biosynthesis of iso-fatty acids in myxobacteria. Org Biomol Chem 2005, 3, 2824–2831. [Google Scholar]
- Thiel, V; Vilchez, R; Szatjer, HI; Wagner-Döbler, I; Schulz, S. Identification, Quantification, and Determination of the Absolute Configuration of the Bacterial Quorum-Sensing Signal Autoinducer-2 by Gas Chromatography-Mass Spectrometry. Chembiochem 2009, 10, 479–485. [Google Scholar]
- Sobik, P; Grunenberg, J; Böröczky, K; Laatsch, H; Wagner-Döbler, I; Schulz, S. Identification, synthesis, and conformation of tri- and tetrathiacycloalkanes from marine bacteria. J Org Chem 2007, 72, 3776–3782. [Google Scholar]
- Dickschat, JS; Nawrath, T; Thiel, V; Kunze, B; Müller, R; Schulz, S. Biosynthesis of the Off-Flavor 2-Methylisoborneol by the Myxobacterium Nannocystis exedens. Angew Chem Int Ed 2007, 46, 8287–8290. [Google Scholar]
- Schulz, S; Dickschat, JS; Müller, R. University of Braunschweig—Institute of Technology, Braunschweig, Germany. In Volatile compounds from Myxobacteria; 2006; unpublished work. [Google Scholar]
- Andersen, JB; Heydorn, A; Hentzer, M; Eberl, L; Geisenberger, O; Christensen, BB; Molin, S; Givskov, M. gfp-Based N-Acyl Homoserine-Lactone Sensor Systems for Detection of Bacterial Communication. Appl Environ Microbiol 2001, 67, 575–585. [Google Scholar]
- Riedel, K; Hentzer, M; Geisenberger, O; Huber, B; Steidle, A; Wu, H; Hoiby, N; Givskov, M; Molin, S; Eberl, L. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 2001, 147, 3249–3262. [Google Scholar]
- Nawrath, T; Dickschat, JS; Kunze, B; Schulz, S. The Biosynthesis of Branched Dialkylpyrazines in Myxobacteria. Chem Biodivers 2010, 7, 2129–2144. [Google Scholar]


| No | Compound | Amount | Asf | Boc | Pyd | Caa | Hna | Scc | tolC | Psa | Kbp | Sta | Mcl | Myp | MIC | Oc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μg | mm | mm | mm | mm | mm | mm | mm | mm | mm | mm | mm | mm | μmol | |||
| 1 | Octanoic acid | 112 | 10i | 20 | 11i | 11i | 0 | 0 | 0 | 0 | 0 | 0 | 480 | (1) | ||
| 2 | 9-Methyl-3-decanol | 156 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 8 | 13 | 20 | 560 | [19] | ||
| 3 | 10-Methylundecan-2-ol | 382 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 420 | [20] | ||
| 4 | 10-Methylundecan-4-olide | 416 | 11 | 14 | 0 | 0 | 14 | 0 | 0 | 8i | 10i | 19 | 430 | [21] | ||
| 5 | 10-Methylundec-2-en-4-olide | 346 | 15 | 14 | 9i | 14 | 17 | 11i | 0 | 9 | 8i | 11 | 363 | [21] | ||
| 6 | 10-Methylundec-3-en-4-olide | 218 | 9i | 9 | 0 | 10 | 13 | 13i | 0 | 8i | 8i | 12 | 230 | [21] | ||
| 7 | 10-Methyldodecan-4-olide | 428 | 8i | 10i | 0 | 0 | 15 | 12i | 0 | 8i | 8i | 13 | 415 | [21] | ||
| 8 | 10-Methyldodec-2-en-4-olide | 140 | 8i | 8 | 0 | 9 | 20 | 0 | 0 | 8 | 8 | 10 | 135 | [21] | ||
| 9 | 10-Methylundecan-5-olide | 250 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 780 | [21] | ||
| 10 | 9-Methylundecan-6-olide | 28 | 0 | 0 | 0 | 0 | 8i | 0 | 0 | 0 | 0 | 18i | 260 | [22] | ||
| 11 | 10-Methylundecan-6-olide | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 280 | [22] | ||
| 12 | 6-Ethyl-3-octanone | 304 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | (2) | ||
| 13 | 9-Methyl-3-decanone | 162 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 590 | [19] | ||
| 14 | 9-Methyl-1-phenyldecan-1-one | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 500 | [20] | |
| 15 | 3-Methyltridecan-4-one | 528 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 510 | [20] | ||
| 16 | 2-Methyltridec-2-en-4-one | 414 | 0 | 0 | 0 | 8i | 0 | 0 | 0 | 0 | 0 | 0 | 405 | [20] | ||
| 17 | 2-Methyl-4-tetradecanone | 488 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 443 | [20] | ||
| 18 | (Z)-13-Methyltetradec-8-en-2-one | 280 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 257 | [23] | ||
| 19 | (Z)-15-Methylhexadec-12-en-2-one | 280 | 8i | 9i | 0 | 9 | 9 | 12i | 0 | 13i | 10 | 13 | 73 | [23] | ||
| 20 | Isopentyl formamide | 524 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [20] | ||
| 21 | Isopentyl acetamide | 386 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [20] | ||
| 22 | 3-Methyl-N-(2-phenylethyl)pentanamide | 290 | 12i | 13i | 15i | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 815 | [24] | ||
| 23 | 4-Methyl-N-(2-phenylethyl)pentanamide | 130 | 10i | 0 | 10i | 0 | 10i | 0 | 0 | 0 | 0 | 0 | 365 | [24] | ||
| 24 | N-(2-phenylethyl)benzamide | 282 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 773 | [24] | ||
| 25 | 2,5-Diisobutylpyrazine | 1322 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | >1000 | [25] | ||
| 26 | 2-Butyl-3,6-dimethylpyrazine | 644 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | >1000 | [23] | ||
| 27 | 2-Isopropenyl-5-isopropylpyrazine | 178 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 680 | [26] | ||
| 28 | 2-Methoxy-3,6-dimethylpyrazine | 217 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 320 | [26] | ||
| 29 | 2-Methoxy-3,5-diisobutylpyrazine | 354 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | >1000 | [26] | ||
| 30 | 2-Methoxy-3,6-diisobutylpyrazine | 354 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 327 | [26] | ||
| 31 | 2-Methoxy-3,6-di-sec-butylpyrazine | 106 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 487 | [26] | ||
| 32 | 2-Methylsulfanyl-3,6-dimethylpyrazine | 916 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 425 | [26] | ||
| 33 | 4-Methylsulfanyl-2-butanol | 220 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [23] | ||
| 34 | S-Methyl 3-(Methylsulfanyl)propanethioate | 1100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [27] | ||
| 35 | S-Methyl benzothioate | 406 | 10i | 11i | 10i | 0 | 8i | 0 | 0 | 0 | 0 | 0 | >1000 | [28] | ||
| 36 | 2-Ethyl-5-isobutylthiophene | 238 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | (2) | ||
| 37 | 3,3,6,6-Tetramethyl-1,2,5-trithiepane | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 705 | [29] | |
| 38 | 3,3,7,7-Tetramethyl-1,2,5-trithiepane | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [29] | |
| 39 | 3,3,8,8-Tetramethyl-1,2,5,6-tetrathiocane | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [29] | |
| 40 | 3,3,7,7-Tetramethyl-1,2,5,6-tetrathiocane | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [29] | |
| 41 | 2-Pentylpyridine | 1198 | 0 | 0 | 0 | 8i | 8 | 0 | 8i | 0 | 0 | 0 | >1000 | (3) | ||
| 42 | Furfuryl isovalerate | 494 | 11i | 15 | 0 | 12 | 8 | 13 | 0 | 13 | 9 | 12 | 62 | [26] | ||
| 43 | 2-Furanmethanol | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [30] | |
| 44 | Tropone | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [27,28] | |
| Common Compounds | ||||||||||||||||
| 45 | 3-Methylbutanol | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [3] | |
| 46 | 2-Phenylethanol | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [21,23,27] | |
| 47 | (2R,3R)-Butanediol | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [3] | |
| 48 | Acetoin | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [3] | |
| 49 | Geosmin | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [21] | |
| 50 | (+)-R-Methylisoborneol | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [21] | |
| 51 | (−)-S-Methylisoborneol | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 240 | ||
| 52 | Dimethyl disulfide | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >1000 | [21] | |
| No | Compound | Conc. | C12-AHL | C6-AHL |
|---|---|---|---|---|
| μg/mL | % | % | ||
| 1 | Octanoic acid | 140 | 23 | 15 |
| 2 | 9-Methyl-3-decanol | 250 | 6 | −198 |
| 3 | 10-Methylundecan-2-ol | 477 | 60 | 3 |
| 4 | 10-Methylundecan-4-olide | 500 | 7 | −550 |
| 5 | 10-Methylundec-2-en-4-olide | 432 | 59 | −40 |
| 6 | 10-Methylundec-3-en-4-olide | 272 | 52 | −16 |
| 7 | 10-Methyldodecan-4-olide | 535 | 71 | −70 |
| 8 | 10-Methyldodec-2-en-4-olide | 175 | 61 | −32 |
| 9 | 10-Methylundecan-5-olide | 312 | 82 | 15 |
| 10 | 9-Methylundecan-6-olide | 35 | 20 | 12 |
| 11 | 10-Methylundecan-6-olide | 37 | 26 | 41 |
| 12 | 6-Ethyl-3-octanone | 250 | −15 | −39 |
| 13 | 9-Methyl-3-decanone | 250 | 10 | −115 |
| 14 | 9-Methyl-1-phenyldecan-1-one | 500 | 1 | −27 |
| 15 | 3-Methyltridecan-4-one | 660 | 59 | −3 |
| 16 | 2-Methyltridec-2-en-4-one | 517 | 52 | −9 |
| 17 | 2-Methyl-4-tetradecanone | 610 | 46 | 53 |
| 18 | (Z)-13-Methyltetradec-8-en-2-one | 315 | 56 | −18 |
| 19 | (Z)-15-Methylhexadec-12-en-2-one | 315 | 82 | 17 |
| 20 | Isopentyl formamide | 655 | 26 | 34 |
| 21 | Isopentyl acetamide | 482 | 15 | 31 |
| 22 | 3-Methyl-N-(2-phenylethyl)pentanamide | 362 | 12 | 18 |
| 23 | 4-Methyl-N-(2-phenylethyl)pentanamide | 162 | −13 | 19 |
| 24 | N-(2-phenylethyl)benzamide | 350 | 28 | 49 |
| 25 | 2,5-Diisobutylpyrazine | 500 | −5 | −94 |
| 26 | 2-Butyl-3,6-dimethylpyrazine | 500 | 13 | 40 |
| 27 | 2-Isopropenyl-5-isopropylpyrazine | 222 | 35 | 72 |
| 28 | 2-Methoxy-3,6-dimethylpyrazine | 500 | 40 | 60 |
| 29 | 2-Methoxy-3,5-diisobutylpyrazine | 250 | 10 | −92 |
| 30 | 2-Methoxy-3,6-diisobutylpyrazine | 500 | 26 | −102 |
| 31 | 2-Methoxy-3,6-di-sec-butylpyrazine | 132 | 33 | 36 |
| 32 | 2-Methylsulfanyl-3,6-dimethylpyrazine | 500 | 5 | 31 |
| 33 | 4-Methylsulfanyl-2-butanol | 275 | 26 | 32 |
| 34 | S-Methyl 3-(Methylsulfanyl)propanethioate | 1375 | 54 | 85 |
| 275 | 40 | |||
| 35 | S-Methyl benzothioate | 500 | −2 | 46 |
| 36 | 2-Ethyl-5-isobutylthiophene | 250 | −25 | −180 |
| 37 | 3,3,6,6-Tetramethyl-1,2,5-trithiepane | 500 | −22 | 51 |
| 38 | 3,3,7,7-Tetramethyl-1,2,5-trithiepane | 500 | 25 | 18 |
| 39 | 3,3,8,8-Tetramethyl-1,2,5,6-tetrathiocane | 500 | 46 | 39 |
| 40 | 3,3,7,7-Tetramethyl-1,2,5,6-tetrathiocane | 250 | 30 | 20 |
| 41 | 2-Pentylpyridine | 1497 | 29 | 78 |
| 42 | Furfuryl isovalerate | 617 | 56 | 84 |
| 43 | 2-Furylmethanol | 500 | 22 | 46 |
| 44 | Tropone | 500 | 29 | 36 |
| Common Compounds | ||||
| 45 | 3-Methylbutanol | 500 | 13 | 61 |
| 250 | 14 | |||
| 46 | 2-Phenylethanol | 500 | 45 | 69 |
| 250 | 23 | |||
| 50 | 31 | |||
| 47 | (2R,3R)-Butanediol | 500 | −14 | 7 |
| 48 | Acetoin | 500 | 23 | 23 |
| 49 | Geosmin | 500 | 13 | 21 |
| 50 | (+)-R-Methylisoborneol | 250 | 3 | 69 |
| 25 | 17 | |||
| 51 | (−)-S-Methylisoborneol | 500 | 12 | 75 |
| 250 | 24 | |||
| 50 | 10 | |||
| 52 | Dimethyl disulfide | 500 | 17 | 26 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schulz, S.; Dickschat, J.S.; Kunze, B.; Wagner-Dobler, I.; Diestel, R.; Sasse, F. Biological Activity of Volatiles from Marine and Terrestrial Bacteria. Mar. Drugs 2010, 8, 2976-2987. https://doi.org/10.3390/md8122976
Schulz S, Dickschat JS, Kunze B, Wagner-Dobler I, Diestel R, Sasse F. Biological Activity of Volatiles from Marine and Terrestrial Bacteria. Marine Drugs. 2010; 8(12):2976-2987. https://doi.org/10.3390/md8122976
Chicago/Turabian StyleSchulz, Stefan, Jeroen S. Dickschat, Brigitte Kunze, Irene Wagner-Dobler, Randi Diestel, and Florenz Sasse. 2010. "Biological Activity of Volatiles from Marine and Terrestrial Bacteria" Marine Drugs 8, no. 12: 2976-2987. https://doi.org/10.3390/md8122976
APA StyleSchulz, S., Dickschat, J. S., Kunze, B., Wagner-Dobler, I., Diestel, R., & Sasse, F. (2010). Biological Activity of Volatiles from Marine and Terrestrial Bacteria. Marine Drugs, 8(12), 2976-2987. https://doi.org/10.3390/md8122976