Intramolecular Modulation of Serine Protease Inhibitor Activity in a Marine Cyanobacterium with Antifeedant Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Feeding Experiments
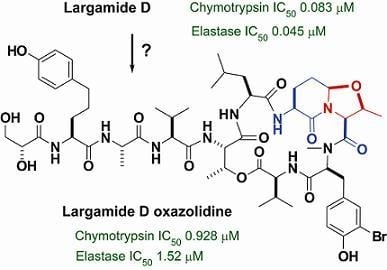
2.2. Isolation and Structure Determination of Largamide D Oxazolidine (1)
2.3. Serine Protease Inhibition Study
2.4. Conclusion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Marine Cyanobacterial Samples
3.3. Feeding Experiments
3.4. Extraction and Isolation
3.5. Largamide D oxazolidine (1)
3.6. Protease Inhibition Assays
Acknowledgements
- Supplementary Material Available: NMR spectra of compound 1.
References
- Cardellina, JH; Marner, FJ; Moore, RE. Seaweed dermatitis: structure of lyngbyatoxin A. Science 1979, 204, 193–195. [Google Scholar]
- Botes, DP; Kruger, H; Viljoen, CC. Isolation and characterization of four toxins from the blue-green alga, Microcystis aeruginosa. Toxicon 1982, 20, 945–954. [Google Scholar]
- Ohtani, I; Moore Moore, RE; Maria, TC. Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc 1992, 114, 7941–7942. [Google Scholar]
- Carmichael, WW. Cyanobacteria secondary metabolites–The cyanotoxins. J. Appl. Bacteriol 1992, 72, 445–459. [Google Scholar]
- Humpage, A. Hudnell, HK, Ed.; Toxin types, toxicokinetics and toxicodynamics. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs, Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 619, pp. 383–415. [Google Scholar]
- Paul, VJ; Arthur, KE; Ritson-Williams, R; Ross, C; Sharp, K. Chemical defenses: from compounds to communities. Biol. Bull 2007, 213, 226–251. [Google Scholar]
- Nagle, DG; Paul, VJ. Chemical defense of a marine cyanobacterial bloom. J. Exp. Mar. Biol. Ecol 1998, 225, 29–38. [Google Scholar]
- Nagle, DG; Paul, VJ. Production of secondary metabolites by filamentous tropical marine cyanobacteria: ecological functions of the compounds. J. Phycol 1999, 35, 1412–1421. [Google Scholar]
- Capper, A; Cruz-Rivera, E; Paul, VJ; Tibbetts, IR. Chemical deterrence of a marine cyanobacterium against sympatric and non-sympatric consumers. Hydrobiologia 2006, 553, 319–326. [Google Scholar]
- Sharp, K; Arthur, KE; Gu, L; Ross, C; Harrison, G; Gunasekera, SP; Meickle, T; Matthew, S; Luesch, H; Thacker, RW; Sherman, DH; Paul, VJ. Phylogenetic and chemical diversity of three chemotypes of bloom-forming Lyngbya species (cyanobacteria: Oscillatoriales) from reefs of southeastern Florida. Appl. Environ. Microbiol 2009, 75, 2879–2888. [Google Scholar]
- Linington, RG; Edwards, DJ; Shuman, CF; McPhail, KL; Matainaho, T; Gerwick, WH. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod 2008, 71, 22–27. [Google Scholar]
- Gunasekera, SP; Miller, MW; Kwan, JC; Luesch, H; Paul, VJ. Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J. Nat. Prod 2010, 73, 459–462. [Google Scholar]
- Kwan, JC; Taori, K; Paul, VJ; Luesch, H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar]
- Banker, R; Carmeli, S. Inhibitors of serine proteases from a waterbloom of the cyanobacterium Microcystis sp. Tetrahedron 1999, 55, 1083–10844. [Google Scholar]
- Grach-Pogrebinsky, O; Sedmak, BC. Protease inhibitors from a Slovenian Lake Bled toxic waterbloom of the cyanobacterium Planktothrix rubescens. Tetrahedron 2003, 59, 8329–8336. [Google Scholar]
- Radau, G. Cyanopeptides: A new and nearly inexhaustible natural resource for the design and structure-activity relationship studies of the new inhibitors of trypsin-like serine proteases. Curr. Enz. Inhib 2005, 1, 295–307. [Google Scholar]
- Baumann, HI; Keller, S; Wolter, FE; Nicholson, GJ; Jung, G; Süssmuth, RD; Friedrich, J. Planktocyclin, a cyclooctapeptide protease inhibitor produced by the freshwater cyanobacterium Planktothrix rubescens. J. Nat. Prod 2007, 70, 1611–1615. [Google Scholar]
- Nakano, Y; Shirai, M; Mori, N; Nakano, M. Neutralization of microcystin shock in mice by tumor necrosis factor alpha antiserum. Appl. Environ. Microbiol 1991, 57, 327–330. [Google Scholar]
- Schatz, D; Keren, Y; Vardl, A; Sukenlk, A; Carmeli, S; Börner, T; Dittmann, E; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol 2007, 9, 965–970. [Google Scholar]
- Paul, VJ; Thacker, RW; Banks, K; Golubic, S. Benthic cyanobacterial bloom impacts the reefs of South Florida (Broward county, USA). Coral Reefs 2005, 24, 693–697. [Google Scholar]
- Matthew, S; Ross, C; James, RR; Paul, VJ; Luesch, H. Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod 2007, 70, 124–127. [Google Scholar]
- Taori, K; Matthew, S; Ross, C; James, RR; Paul, VJ; Luesch, H. Lyngbyastatin 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod 2007, 70, 1593–1600. [Google Scholar]
- Matthew, S; Ross, C; Paul, VJ; Luesch, H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron 2008, 64, 4081–4089. [Google Scholar]
- Plaza, A; Bewley, CA. Largamides A–H, Unusual cyclic peptides from the marine cyanobacterium Oscillatoria sp. J. Org. Chem 2006, 71, 6898–6907. [Google Scholar]
- Gunasekera, SP; Ritson-Williams, R; Paul, VJ. Carriebowmide, a new cyclodepsipeptide from the marine cyanobacterium Lyngbya polychroa. J. Nat. Prod 2008, 71, 2060–2063. [Google Scholar]
- Cruz-Rivera, E; Paul, VJ. Chemical deterrence of a cyanobacterial metabolite against generalized and specialized grazers. J. Chem. Ecol 2007, 33, 213–217. [Google Scholar]
- Thacker, RW; Nagle, DG; Paul, VJ. Effects of repeated exposures to marine cyanobacterial secondary metabolites on feeding by juvenile rabbitfish and parrotfish. Mar. Ecol. Prog. Ser 1997, 147, 21–29. [Google Scholar]
- Capper, A; Paul, VJ. Grazer interactions with four species of Lyngbya in southeast Florida. Harmful Algae 2008, 7, 717–728. [Google Scholar]
- He, H-P; Shen, Y-M; Zhang, J-X; Zuo, G-Y; Hao, X-J. New diterpene alkaloids from the roots of Spiraea japonica. J. Nat. Prod 2001, 64, 379–380. [Google Scholar]
- Kunimoto, S; Lu, J; Esumi, H; Yamazaki, Y; Kinoshita, N; Honma, Y; Hamada, M; Ohsono, M; Ishizuka, M; Takeuchi, T. Kigamicins, novel antitumor antibiotics. I. Taxonomy, isolation, physico-chemical properties and biological activities. J. Antibiot 2003, 56, 1004–1011. [Google Scholar]
- Hopp, DC; Milanowski, DJ; Rhea, J; Jacobsen, D; Rabenstein, J; Smith, C; Romari, K; Clarke, M; Francis, L; Irigoyen, M; Luche, M; Carr, GJ; Mocek, U. Citreamicins with potent gram-positive activity. J. Nat. Prod 2008, 71, 2032–2035. [Google Scholar]
- Asai, Y; Nonaka, N; Suzuki, S-I; Nishio, M; Takahashi, K; Shima, H; Ohmori, K; Ohnuki, T; Komatsubara, S. TMC-66, a new endothelin converting enzyme inhibitor produced by Streptomyces sp. A5008. J. Antibiot 1999, 52, 607–612. [Google Scholar]
- Matthew, S; Paul, VJ; Luesch, H. Tiglicamides A–C, Cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry 2009, 70, 2058–2063. [Google Scholar]





| Unita | 1 (DMF-d7) δH (J in Hz) | δC | COSY | HMBC | 1 (methanol-d4) δH (J in Hz) | δC | 2 (methanol-d4) δH (J in Hz) | δC | |
|---|---|---|---|---|---|---|---|---|---|
| Val-1 | 1 | 171.4 | 173.6 | 174.8 | |||||
| 2 | 4.53, m | 56.8 | H-3, NH | 1, 3, 4, 5, 1 (N-Me-Br-Tyr) | 4.72, m | 58.1 | 4.49, d (7.4) | 58.9 | |
| 3 | 2.32, m | 31.1 | H-2, H3-4, H3-5 | 2, 4, 5 | 2.38, m | 32.7 | 2.15, m | 31.8 | |
| 4 | 0.92, d (7.0) | 19.0 | H-3 | 2, 3, 5 | 0.92, d (7.2) | 18.8 | 0.93, d (6.9) | 18.9 | |
| 5 | 0.86, d (7.0) | 17.5 | H-3 | 2, 3, 4 | 0.89, d (7.2) | 18.0 | 0.99, d (6.9) | 19.8 | |
| NH | 6.76, d (8.3) | H-2 | |||||||
| N-Me- Br-Tyr | 1 | 169.2 | 170.9 | 171.4 | |||||
| 2 | 5.71, dd (11.5, 2.5) | 62.5 | H-3a/3b | 1 | 5.75, dd (11.5, 2.4) | 63.9 | 5.06, dd (11.5, 3.1) | 62.6 | |
| 3a | 3.34, dd (−14.3, 11.5) | 32.4 | H-2, H-3b H-2, H-3a | 2, 4 | 3.28, dd (−14.4, 2.4) | 33.5 | 3.40, dd (−14.6, 3.1) | 33.8 | |
| 3b | 2.89, dd (−14.3, 2.5) | 2.84, dd (−14.4, 11.5) | 2.80, m | ||||||
| 4 | 130.5 | 131.4 | 130.4 | ||||||
| 5 | 7.44, d (1.9) | 133.8 | 4, 6, 7, 9 | 7.32, d (1.2) | 134.8 | 7.34, d (2.0) | 134.8 | ||
| 6 | 109.3 | 111.2 | 110.8 | ||||||
| 7 | 153.6 | 154.7 | 154.4 | ||||||
| 8 | 7.03, d (8.2) | 116.7 | H-9 | 4, 6, 7, 9 | 6.83, d (8.3) | 117.7 | 6.87, d (8.2) | 117.6 | |
| 9 | 7.13, dd (8.2, 1.9) | 129.9 | H-8 | 5, 8 | 7.02, dd (8.3, 1.2) | 130.8 | 7.17, dd (8.2, 2.0) | 130.7 | |
| N-Me | 2.92, s | 30.8 | 1, 2, 1 (Thr-1) | 2.90, s | 31.8 | 2.87, s | 31.2 | ||
| Thr-1a | 1 | 169.7 | 172.0 | 173.0 | |||||
| 2 | 4.04, d (3.4) | 59.5 | H-3 | 1, 3, 2 (Ahp), H-6 (Ahp) | 3.96, d (2.3) | 61.0 | 4.57, d (6.8) | 55.9 | |
| 3 | 4.36, qd (6.4, 3.4) | 76.4 | H3-4 | 1, 2, 4 | 4.38, dq (6.2, 2.3) | 77.4 | 3.74, m | 66.6 | |
| 4 | 0.71, d (6.4) | 19.4 | H-3 | 2, 3 | 0.70, d (6.2) | 20.5 | 0.57, d (6.2) | 19.6 | |
| Ahpa | 2 | 168.8 | 170.5 | 171.3 | |||||
| 3 | 4.63, ddd (12.4, 8.7, 4.9) | 50.3 | H-4a/4b, NH | 2, 4, 5, 6 | 4.63, dd (9.9, 5.3) | 51.7 | 4.64, dd (12.4, 6.4) | 50.7 | |
| 4a | 1.95, m | 24.6 | H-3, H-4b, H-5a/5b | 2, 3, 5, 6 | 1.97, m | 25.3 | 2.82, m | 22.0 | |
| 4b | 1.88, m | H-3, H-4a, H-5a/5b | 1.93, m | 1.88, m | |||||
| 5a | 2.38, m | 27.9 | H-4a/4b, H-5b, H-6 | 3, 4, 6 | 2.40, m | 29.2 | 2.04, m | 30.6 | |
| 5b | 1.68, m | H-4a/4b, H-5a, H-6 | 1.66, m | 1.87, m | |||||
| 6 | 5.01, dd (8.5, 6.2) | 87.4 | H-5a/5b | 2, 4, 5 | 5.03, dd (8.5, 6.5) | 88.6 | 5.33, brs | 76.9 | |
| NH | 7.22, d (8.7) | H-3 | 3, 1 (Leu) | ||||||
| Leu | 1 | 171.7 | 174.6 | 174.4 | |||||
| 2 | 4.49, m | 51.4 | H-3a/3b | 1, 3 | 4.58, dd (11.0, 3.0) | 52.6 | 4.56, dd (8.5, 3.3) | 52.9 | |
| 3a | 1.84, m | 40.6 | H-2, H-3b | 1, 2, 4 | 1.88, m | 41.3 | 1.98, ddd (12.6, 12.6, 3.3) | 40.2 | |
| 3b | 1.47, m | H-2, H-3a | 1.47, m | 1.55, m | |||||
| 4 | 1.61, m | 24.5 | H-3a/3b, H3-5, H3-6 | 2, 3, 5, 6 | 1.56, m | 25.8 | 1.65, m | 25.8 | |
| 5 | 0.87, d (6.6) | 22.9 | H-4 | 4 | 0.91, d (6.0) | 23.8 | 0.87, d (6.8) | 20.0 | |
| 6 | 0.82, d (6.6) | 20.8 | H-4 | 4 | 0.83, d (6.0) | 21.5 | 0.97, d (6.7) | 23.7 | |
| NH | 8.50, d (8.5) | H-2 | 2, 1 (Thr-2) | ||||||
| Thr-2 | 1 | 169.7 | 171.1 | 170.9 | |||||
| 2 | 4.92, dd (9.0, 1.7) | 55.4 | NH | 1, 3, 1 (Val-2) | 4.72, m | 56.7 | 4.78, d (1.2) | 56.3 | |
| 3 | 5.78, qd (6.0, 1.7) | 72.1 | H3-4 | 1, 2, 4, 1 (Val-1) | 5.64, qd (6.5, 1.4) | 73.2 | 5.59, qd (6.5, 1.2) | 73.4 | |
| 4 | 1.33, d (6.0) | 16.6 | H-3 | 2, 3 | 1.30, d (6.5) | 17.7 | 1.36, d (6.5) | 18.1 | |
| NH | 8.25, d (9.0) | H-2 | 2, 1 (Val-2) | ||||||
| Val-2 | 1 | 171.7 | 173.8 | 173.9 | |||||
| 2 | 4.53, dd (8.6, 5.5) | 58.0 | H-3, NH | 1, 3, 4, 5 | 4.30, d (7.8) | 60.2 | 4.39, d (7.4) | 59.7 | |
| 3 | 2.17, m | 31.2 | H-2, H3-4, H3-5 | 2, 4, 5 | 2.09, dd (7.8, 6.8) | 32.0 | 2.17, m | 31.9 | |
| 4 | 0.90, d (7.0) | 18.5 | H-3 | 2, 3, 5 | 0.94, d (6.8) | 18.8 | 0.98, d (6.9) | 18.5 | |
| 5 | 0.87, d (7.0) | 17.5 | H-3 | 2, 3, 4 | 0.90, d (6.8) | 19.4 | 0.98, d (6.9) | 19.8 | |
| NH | 7.76, d (8.6) | H-2 | 2, 1 (Ala) | ||||||
| Ala | 1 | 172.0b | 174.8 | 174.4 | |||||
| 2 | 4.49, dq (9.0, 7.2) | 48.9 | H-3, NH | 1, 3 | 4.38, q (7.0) | 49.0 | 4.45, m | 49.9 | |
| 3 | 1.30, d (7.2) | 17.0 | H-2 | 1, 2 | 1.33, d (7.0) | 17.8 | 1.38, d (7.2) | 17.6 | |
| NH | 8.26, d (9.0) | H-2 | 2, 1 (Ahppa) | ||||||
| Ahppa | 1 | 171.9b | 174.1 | 173.5 | |||||
| 2 | 4.52, m | 52.6 | H-3a/3b, NH | 1, 3, 4, NH | 4.41, m | 54.4 | 4.46, m | 53.9 | |
| 3a | 1.89, m | 32.4 | H-2, H-3b, H-4a/4b | 2, 4, 5 | 1.87, m | 32.7 | 1.91, m | 32.6 | |
| 3b | 1.73, m | H-2, H-3a, H-4a/4b | 1.69, m | 1.73, m | |||||
| 4a | 1.68, m | 28.2 | H-3a/3b, H-4b | 2, 3, 5, 6 | 1.68, m | 29.0 | 1.70, m | 28.8 | |
| 4b | 1.62, m | H-3a/3b, H-4a | 1.63, m | 1.66, m | |||||
| 5 | 2.52, m | 34.2 | H-4a/4b | 3, 4, 6 | 2.54, m | 35.6 | 2.57, t (7.3) | 35.2 | |
| 6 | 132.6 | 134.0 | 133.6 | ||||||
| 7/11 | 7.03, d (8.3) | 129.8 | H-8/10 | 6, 8/10, 9 | 7.00, d (8.4) | 130.4 | 7.02, dd (8.2, 1.8) | 130.2 | |
| 8/10 | 6.76, d (8.3) | 115.2 | H-7/11 | 7/11, 9 | 6.67, d (8.4, 2.4) | 116.1 | 6.70, dd (8.2, 1.8) | 115.8 | |
| 9 | 156.0 | 7/11, 8/10 | 156.5 | 156.2 | |||||
| 9-OH | 9.36, s | 8/10, 9 | |||||||
| NH | 7.87, d (7.6) | H-2 | 1, 2, 1 (Ga) | ||||||
| Ga | 1 | 172.4 | 175.4 | 174.9 | |||||
| 2 | 4.11, m | 73.5 | H-3a/3b | 1, 3 | 4.11, d (3.5) | 74.2 | 4.13, t (3.8) | 73.9 | |
| 2-OH | c | ||||||||
| 3a | 3.76, dd (−10.9, 3.5) | 64.7 | H-2, H-3b | 1, 2 | 3.78, 2H, d (3.5) | 65.6 | 3.79, 2H, d (3.8) | 65.2 | |
| 3b | 3.70, dd (−10.9, 5.6) | H-2, H-3a |
| Chymotrypsin | Elastase | |
|---|---|---|
| Largamide D (2) | 0.083 ± 0.008 | 0.045 ± 0.003 |
| Largamide D oxazolidine (1) | 0.928 ± 0.093 | 1.52 ± 0.08 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matthew, S.; Ratnayake, R.; Becerro, M.A.; Ritson-Williams, R.; Paul, V.J.; Luesch, H. Intramolecular Modulation of Serine Protease Inhibitor Activity in a Marine Cyanobacterium with Antifeedant Properties. Mar. Drugs 2010, 8, 1803-1816. https://doi.org/10.3390/md8061803
Matthew S, Ratnayake R, Becerro MA, Ritson-Williams R, Paul VJ, Luesch H. Intramolecular Modulation of Serine Protease Inhibitor Activity in a Marine Cyanobacterium with Antifeedant Properties. Marine Drugs. 2010; 8(6):1803-1816. https://doi.org/10.3390/md8061803
Chicago/Turabian StyleMatthew, Susan, Ranjala Ratnayake, Mikel A. Becerro, Raphael Ritson-Williams, Valerie J. Paul, and Hendrik Luesch. 2010. "Intramolecular Modulation of Serine Protease Inhibitor Activity in a Marine Cyanobacterium with Antifeedant Properties" Marine Drugs 8, no. 6: 1803-1816. https://doi.org/10.3390/md8061803
APA StyleMatthew, S., Ratnayake, R., Becerro, M. A., Ritson-Williams, R., Paul, V. J., & Luesch, H. (2010). Intramolecular Modulation of Serine Protease Inhibitor Activity in a Marine Cyanobacterium with Antifeedant Properties. Marine Drugs, 8(6), 1803-1816. https://doi.org/10.3390/md8061803