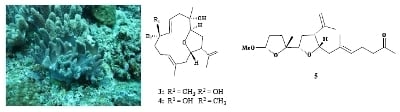
Cembranoids with 3,14-Ether Linkage and a Secocembrane with Bistetrahydrofuran from the Dongsha Atoll Soft Coral Lobophytum sp.
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.4. Cytotoxicity Testing
3.5. Molecular Mechanics Calculations
Acknowledgements
- Samples Availability: Not available.
References
- Blunt, JW; Copp, BR; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat. Prod. Rep 2011, 28, 196–268. [Google Scholar]
- Su, J-H; Ahmed, AF; Sung, P-J; Chao, C-H; Kuo, Y-H; Sheu, J-H. Manaarenolides A–I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod 2006, 69, 1134–1139. [Google Scholar]
- Lu, Y; Huang, C-Y; Lin, Y-F; Wen, Z-H; Su, J-H; Kuo, Y-H; Chiang, MY; Sheu, J-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod 2008, 71, 1754–1759. [Google Scholar]
- Ahmed, AF; Tai, S-H; Wen, Z-H; Su, J-H; Wu, Y-C; Hu, W-P; Sheu, J-H. A C-3 methylated isocembranoid and 10-oxocembranoids from a Formosan soft coral, Sinularia grandilobata. J. Nat. Prod 2008, 71, 946–951. [Google Scholar]
- Ahmed, AF; Wen, Z-H; Su, J-H; Hsieh, Y-T; Wu, Y-C; Hu, W-P; Sheu, J-H. Oxygenated cembranoids from a Formosan soft coral Sinularia gibberosa. J. Nat. Prod 2008, 71, 179–185. [Google Scholar]
- Lu, Y; Su, J-H; Hsieh, C-H; Liu, Y-C; Kuo, Y-H; Wen, Z-H; Hsu, C-H; Sheu, J-H. Cembranoids from the soft corals Sinularia granosa and Sinularia querciformis. Chem. Pharm. Bull 2010, 58, 464–466. [Google Scholar]
- Chen, B-W; Chao, C-H; Su, J-H; Huang, C-Y; Dai, C-F; Wen, Z-H; Sheu, J-H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett 2010, 44, 5764–5766. [Google Scholar]
- Lin, S-T; Wang, S-K; Cheng, S-Y; Duh, C-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod 2009, 72, 152–155. [Google Scholar]
- Cheng, S-Y; Wen, Z-H; Wang, S-K; Chiou, S-F; Hsu, C-H; Dai, C-F; Chiang, MY; Duh, C-Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett 2009, 11, 3012–3014. [Google Scholar]
- Chao, C-H; Wen, Z-H; Wu, Y-C; Yeh, H-C; Sheu, J-H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod 2008, 71, 1819–1824. [Google Scholar]
- Huang, H-C; Ahmed, AF; Su, J-H; Wu, Y-C; Chiang, MY; Sheu, J-H. Crassocolides A–F, cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule. J. Nat. Prod 2006, 69, 1554–1559. [Google Scholar]
- Huang, H-C; Chao, C-H; Kuo, Y-H; Sheu, J-H. Crassocolides G-M, cembranoids from the Formosan soft coral Sarcophyton crassocaule. Chem. Biodiv 2009, 6, 1232–1242. [Google Scholar]
- Cheng, Y-B; Shen, Y-C; Kuo, Y-H; Khalil, AT. Cembrane diterpenoids from the Taiwanese soft coral Sarcophyton stolidotum. J. Nat. Prod 2008, 71, 1141–1145. [Google Scholar]
- Cheng, S-Y; Wang, S-K; Chiou, S-F; Hsu, C-H; Dai, C-F; Chiang, MY; Duh, C-Y. Cembranoids from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod 2010, 73, 197–203. [Google Scholar]
- Lin, W-Y; Su, J-H; Lu, Y; Wen, Z-H; Dai, C-F; Kuo, Y-H; Sheu, J-H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem 2010, 18, 1936–1941. [Google Scholar]
- Lin, W-Y; Lu, Y; Su, J-H; Wen, Z-H; Dai, C-F; Kuo, Y-H; Sheu, J-H. Bioactive cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar]
- Sheu, J-H; Wang, G-H; Sung, P-J; Duh, C-Y; Chiang, MY. Pachyclavulariolides G–L and secopachyclavulariaenone A, seven novel diterpenoids from the soft coral Pachyclavularia violacea. Tetrahedron 2001, 57, 7639–7648. [Google Scholar]
- Sheu, J-H; Wang, G-H; Duh, C-Y; Soong, K. Pachyclavulariolides M-R, six novel diterpenoids from a Taiwanese soft coral Pachyclavularia violacea. J. Nat. Prod 2003, 66, 662–666. [Google Scholar]
- Lu, Y; Lin, Y-C; Wen, Z-H; Su, J-H; Sung, P-J; Hsu, C-H; Kuo, Y-H; Chiang, MY; Sheu, J-H. Steroid and cembranoids from the Dongsha Atoll soft coral Lobophytum. Tetrahedron 2010, 66, 7129–7135. [Google Scholar]
- Ravi, BN; Faulkner, J. Cembranoid diterpenes from a South Pacific soft coral. J. Org. Chem 1978, 43, 2127–2131. [Google Scholar]
- Duh, C-Y; Hou, R-S. Cytotoxic cembranoids from the soft corals Sinularia gibberosa and Sarcophyton trocheliophorum. J. Nat. Prod 1996, 59, 595–598. [Google Scholar]
- Scudiero, DA; Shoemaker, RH; Paull, KD; Monks, A; Tierney, S; Nofziger, TH; Currens, MJ; Seniff, D; Boyd, MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 1988, 48, 4827–4833. [Google Scholar]
 ) and HMBC (→) correlations of 1, 3 and 5.
) and HMBC (→) correlations of 1, 3 and 5.




| C# | 1 a | 2 a | 3 a | 4 b | 5 b |
|---|---|---|---|---|---|
| 1 | 50.2 (CH) c | 49.0 (CH) | 49.3 (CH) | 49.3 (CH) | 49.8 (CH) |
| 2 | 29.1 (CH2) | 27.4 (CH2) | 26.7 (CH2) | 26.7 (CH2) | 30.9 (CH2) |
| 3 | 77.5 (CH) | 77.6 (CH) | 77.8 (CH) | 77.6 (CH) | 82.2 (CH) |
| 4 | 74.5 (C) | 74.2 (C) | 74.6 (C) | 74.7 (C) | 86.6 (C) |
| 5 | 39.1 (CH2) | 38.6 (CH2) | 42.5 (CH2) | 43.3 (CH2) | 31.9 (CH2) |
| 6 | 23.8 (CH2) | 21.5 (CH2) | 118.9 (CH) | 121.8 (CH) | 33.3 (CH2) |
| 7 | 64.7 (CH) | 126.6 (CH) | 142.7 (CH) | 141.5 (CH) | 105.6 (CH) |
| 8 | 60.3 (C) | 132.8 (C) | 73.6 (C) | 72.6 (C) | 208.9 (C) |
| 9 | 38.1 (CH2) | 38.2 (CH2) | 44.4 (CH2) | 43.7 (CH2) | 43.7 (CH2) |
| 10 | 23.9 (CH2) | 24.4 (CH2) | 23.5 (CH2) | 22.2 (CH2) | 22.5 (CH2) |
| 11 | 126.5 (CH) | 127.1 (CH) | 129.4 (CH) | 129.6 (CH) | 124.2 (CH) |
| 12 | 133.0 (C) | 131.9 (C) | 130.9 (C) | 130.8 (C) | 134.2 (C) |
| 13 | 40.2 (CH2) | 39.3 (CH2) | 38.9 (CH2) | 38.8 (CH2) | 39.7 (CH2) |
| 14 | 78.5 (CH) | 76.7 (CH) | 76.0 (CH) | 76.0 (CH) | 80.3 (CH) |
| 15 | 141.6 (C) | 142.4 (C) | 142.2 (C) | 142.3 (C) | 144.0 (C) |
| 16 | 111.3 (CH2) | 111.0 (CH2) | 111.2 (CH2) | 111.1 (CH2) | 112.2 (CH2) |
| 17 | 25.0 (CH3) | 23.5 (CH3) | 23.5 (CH3) | 23.5 (CH3) | 22.5 (CH3) |
| 18 | 24.6 (CH3) | 23.1 (CH3) | 21.6 (CH3) | 21.9 (CH3) | 24.2 (CH3) |
| 19 | 19.8 (CH3) | 16.3 (CH3) | 29.6 (CH3) | 28.3 (CH3) | 29.9 (CH3) |
| 20 | 17.3 (CH3) | 15.4 (CH3) | 15.4 (CH3) | 15.5 (CH3) | 16.5 (CH3) |
| OMe | 54.3 (CH3) |
| 1 a | 2 a | 3 a | 4 b | 5 b | |
|---|---|---|---|---|---|
| 1 | 2.77 dt (8.8, 8.0) c | 2.73 dt (11.2, 7.2) | 2.73 dt (8.0, 8.8) | 2.74 dt (9.0, 8.5) | 2.78 dt (7.5, 8.5) |
| 2 | 2.16 m; 1.92 m | 2.08 m; 1.90 m | 2.04 m; 1.86 m | 2.05 m; 1.86 m | 1.96 m; 1.91 m |
| 3 | 3.98 dd (9.6, 4.4) | 3.97 dd (9.6, 4.5) | 3.82 dd (10.0, 4.8) | 3.82 dd (9.5, 4.5) | 3.98 dd (7.5, 7.5) |
| 5 | 1.97 m; 1.70 m | 1.94 m; 1.53 m | 2.40 dd (14.0, 10.0); 2.05 m | 2.40 dd (14.0, 10.0); 2.10 m | 2.40 dd (14.0, 10.0); 1.94 m |
| 6 | 2.05 m; 1.31 m | 2.25 m; 2.06 m | 5.60 ddd (15.2, 10.0, 5.2) | 5.51 ddd (15.5, 10.0, 5.0) | 2.02 m; 1.94 m |
| 7 | 3.27 d (6.8) | 5.17 dd (6.0, 6.0) | 5.70 d (15.6) | 5.75 d (15.5) | 5.00 d (4.5) |
| 9 | 1.86 m; 1.52 m | 2.14 m; 1.96 m | 1.92 m; 1.58 m | 1.95 m; 1.58 m | 2.45 dd (8.0, 7.0) |
| 10 | 2.21 m; 1.88 m | 2.32 m; 2.04 m | 2.19 m; 2.10 m | 2.56 m; 1.96 m | 2.27 dd (7.5, 7.5) |
| 11 | 5.09 t (6.8) | 4.89 d (8.0) | 4.96 d (9.6) | 4.94 d (10.0) | 5.12 dd (7.0, 6.5) |
| 13 | 1.95 m; 1.68 m | 1.88 m; 1.72 m | 1.91 m; 1.64 m | 1.92 m; 1.64 m | 2.00 m; 1.97 m |
| 14 | 4.37 ddd (12.0, 3.6, 3.6) | 4.36 ddd (11.6, 5.2, 4.8) | 4.33 ddd (11.6, 6.0, 5.2) | 4.33 ddd (12.0, 6.0, 5.5) | 4.14 ddd (9.0, 4.5, 3.5) |
| 16 | 4.87 d (1.6); 4.81 s | 4.85 d (1.2); 4.78 s | 4.86 d (1.6); 4.80 s | 4.86 d (1.0); 4.80 s | 4.83 s; 4.72 s |
| 17 | 1.77 s | 1.75 s | 1.76 s | 1.76 s | 1.75 s |
| 18 | 1.15 s | 1.09 s | 1.11 s | 1.13 s | 1.28 s |
| 19 | 1.24 s | 1.65 s | 1.28 s | 1.37 s | 2.13 s |
| 20 | 1.61 s | 1.57 s | 1.67 s | 1.70 s | 1.65 s |
| OMe | 3.34 s |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hegazy, M.E.F.; Su, J.-H.; Sung, P.-J.; Sheu, J.-H. Cembranoids with 3,14-Ether Linkage and a Secocembrane with Bistetrahydrofuran from the Dongsha Atoll Soft Coral Lobophytum sp. Mar. Drugs 2011, 9, 1243-1253. https://doi.org/10.3390/md9071243
Hegazy MEF, Su J-H, Sung P-J, Sheu J-H. Cembranoids with 3,14-Ether Linkage and a Secocembrane with Bistetrahydrofuran from the Dongsha Atoll Soft Coral Lobophytum sp. Marine Drugs. 2011; 9(7):1243-1253. https://doi.org/10.3390/md9071243
Chicago/Turabian StyleHegazy, Mohamed Elamir F., Jui-Hsin Su, Ping-Jyun Sung, and Jyh-Horng Sheu. 2011. "Cembranoids with 3,14-Ether Linkage and a Secocembrane with Bistetrahydrofuran from the Dongsha Atoll Soft Coral Lobophytum sp." Marine Drugs 9, no. 7: 1243-1253. https://doi.org/10.3390/md9071243