A Microfluidic Device for Continuous Sensing of Systemic Acute Toxicants in Drinking Water
Abstract
:1. Introduction
2. Experimental Section
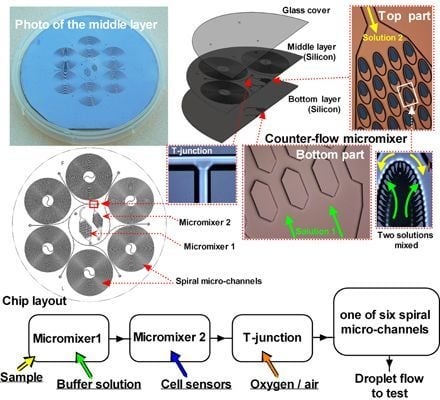
2.1. The Strategy of System Design

2.2. Chip Design
2.3. Chip Fabrication and System Installation


2.4. Cell-Based Toxicity Tests in the LOC
3. Results and Discussion
3.1. The Cell Toxicity Tests on the Chip

3.2. The Design of the Detection Module
3.3. Distinguishing Features and Ethical Issues of the Household LOC System
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Douglas, I. Urban ecology and urban ecosystems: Understanding the links to human health and well-being. Curr. Opin. Environ. Sustain. 2012, 4, 385–392. [Google Scholar] [CrossRef]
- Storey, M.V.; van der Gaag, B.; Burns, B.P. Advances in on-line drinking water quality monitoring and early warning systems. Water Res. 2011, 45, 741–747. [Google Scholar] [CrossRef]
- Lee, A.; Francisque, A.; Najjaran, H.; Rodriguez, M.; Hoorfar, M.; Imran, S.; Sadiq, R. Online monitoring of drinking water quality in a distribution network: A selection procedure for suitable water quality parameters and sensor devices. Int. J. Syst. Assur. Eng. Manag. 2012, 3, 323–337. [Google Scholar] [CrossRef]
- Brack, W. Effect-directed analysis: A promising tool for the identification of organic toxicants in complex mixtures? Anal. Bioanal. Chem. 2003, 377, 397–407. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T.; Yang, Z.; Pires, N.; Hoivik, N. Compatible immuno-NASBA LOC device for quantitative detection of waterborne pathogens: Design and validation. Lab. Chip. 2012, 12, 602–612. [Google Scholar] [CrossRef]
- Figueras, M.J.; Borrego, J.J. New perspectives in monitoring drinking water microbial quality. Int. J. Environ. Res. Public Health 2010, 7, 4179–4202. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T. Multifunctional sample preparation kit and on-chip quantitative nucleic acid sequence-based amplification tests for microbial detection. Anal. Chem. 2012, 84, 8541–8548. [Google Scholar] [CrossRef]
- Curtis, T.M.; Tabb, J.; Romeo, L.; Schwager, S.J.; Widder, M.W.; van der Schalie, W.H. Improved cell sensitivity and longevity in a rapid impedance-based toxicity sensor. J. Appl. Toxicol. 2009, 29, 374–380. [Google Scholar] [CrossRef]
- Ibrahim, F.; Huang, B.; Xing, J.; Gabos, S. Early determination of toxicant concentration in water supply using MHE. Water Res. 2010, 44, 3252–3260. [Google Scholar] [CrossRef]
- Eibl, R.; Kaiser, S.; Lombriser, R.; Eibl, D. Disposable bioreactors: The current state-of-the-art and recommended applications in biotechnology. Appl. Microbiol. Biot. 2010, 86, 41–49. [Google Scholar] [CrossRef]
- Melamed, S.; Elad, T.; Belkin, S. Microbial sensor cell arrays. Curr. Opin. Biotechnol. 2012, 23, 2–8. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, C.; Xu, X.; Hanna, M.; Zhang, X.; Wang, Y.; Dai, H.; Xiao, W. Construction and evaluation of two biosensors based on yeast transcriptional response to genotoxic chemicals. Biosens. Bioelectron. 2013, 44, 138–145. [Google Scholar] [CrossRef]
- Frense, D.; Müller, A.; Beckmann, D. Detection of environmental pollutants using optical biosensor with immobilized algae cells. Sens. Actuators B Chem. 1998, 51, 256–260. [Google Scholar] [CrossRef]
- Rodriguez, M., Jr.; Sanders, C.A.; Greenbaum, E. Biosensors for rapid monitoring of primary-source drinking water using naturally occurring photosynthesis. Biosens. Bioelectron. 2002, 17, 843–849. [Google Scholar] [CrossRef]
- Woutersen, M.; Belkin, S.; Brouwer, B.; van Wezel, A.P.; Heringa, M.B. Are luminescent bacteria suitable for online detection and monitoring of toxic compounds in drinking water and its sources? Anal. Bioanal. Chem. 2011, 400, 915–929. [Google Scholar] [CrossRef]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef]
- ISO 11348-3:2007 Water quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent bacteria test). Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=40518 (accessed on 4 September 2013).
- Zhao, X.; Dong, T. Measurement and Analysis of Vibrio fischeri Cell-Based Microfluidic Device for Personal Health Monitoring. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2433–2436.
- Dong, T.; Su, Q.H.; Yang, Z.C.; Zhang, Y.L.; Egeland, E.B.; Gu, D.D.; Calabrese, P.; Kapiris, M.J.; Karlsen, F.; Minh, N.T.; Wang, K.; Jakobsen, H. A smart fully integrated micromachined separator with soft magnetic micro-pillar arrays for cell isolation. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Pires, N.M.M.; Dong, T. A cascade-like silicon filter for improved recovery of oocysts from environmental waters. Environ. Technol. 2013. Available online: http://www.tandfonline.com/doi/full/10.1080/09593330.2013.851280#.UpcqSuJGa9g (accessed on 28 November 2013). [Google Scholar] [CrossRef]
- Tran-Minh, N.; Dong, T.; Su, Q.H.; Yang, Z.C.; Jakobsen, H.; Karlsen, F. Design and optimization of non-clogging counter-flow microconcentrator for enriching epidermoid cervical carcinoma cells. Biomed. Microdevices 2011, 13, 179–190. [Google Scholar] [CrossRef]
- Zhang, Z.; Perozziello, G.; Boccazzi, P.; Sinskey, A.J.; Geschke, O.; Jensen, K.F. Microbioreactors for bioprocess development. JALA 2007, 12, 143–151. [Google Scholar]
- Pires, N.M.M.; Dong, T. Recovery of Cryptosporidium and Giardia organisms from surface water by counter-flow refining microfiltration. Environ. Technol. 2013, 34, 2541–2551. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Molino, M.; Demarchi, D. Cell-Based Digital Microfluidic Chip for Drug Mixing and Droplets Generation: Design and Simulation. In Proceedings of the 2012 5th International Conference on Biomedical Engineering and Informatics (BMEI), Chongqing, China, 16–18 October 2012; pp. 726–730.
- Zhao, X.; Dong, T. A Household LOC Device for Online Monitoring Bacterial Pathogens in Drinking Water with Green Design Concept. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1708–1711.
- Pires, N.M.; Dong, T.; Hanke, U.; Hoivik, N. Integrated optical microfluidic biosensor using a polycarbazole photodetector for point-of-care detection of hormonal compounds. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, T. A Si/SiGe quantum well based biosensor for direct analysis of exothermic biochemical reaction. J. Micromech. Microeng. 2013, 23. [Google Scholar] [CrossRef]
- Dong, T.; Yang, Z.C.; Su, Q.H.; Nhut, M.T.; Egeland, E.B.; Karlsen, F.; Zhang, Y.L.; Kapiris, M.J.; Jakobsen, H. Integratable non-clogging microconcentrator based on counter-flow principle for continuous enrichment of CaSki cells sample. Microfluid. Nanofluid. 2011, 10, 855–865. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T. Design and fabrication of low-cost 1536-chamber microfluidic microarrays for mood-disorders-related serological studies. Sensors 2013, 13, 14570–14582. [Google Scholar] [CrossRef]
- Hauschild, V.D. Chemical exposure guidelines for deployed military personnel. Drug Chem. Toxicol. 2000, 23, 139–153. [Google Scholar] [CrossRef]
- Galal-Gorchev, H.; Ozolins, G.; Bonnefoy, X. Revision of the WHO guidelines for drinking water quality. Ann. dell’Istituto Super. Sanita 1993, 29, 335–345. [Google Scholar]
- WHO Guidelines for Drinking-Water Quality. Available online: http://www.who.int/water_sanitation_health/dwq/guidelines/en/index.html (accessed on 4 September 2013).
- Backhaus, T.; Scholze, M.; Grimme, L.H. The single substance and mixture toxicity of quinolones to the bioluminescent bacterium Vibrio fischeri. Aquat. Toxicol. 2000, 49, 49–61. [Google Scholar] [CrossRef]
- Gueguen, C.; Gilbin, R.; Pardos, M.; Dominik, J. Water toxicity and metal contamination assessment of a polluted river: The Upper Vistula River (Poland). Appl. Geochem. 2004, 19, 153–162. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- O’Connor, E.; Smeaton, A.F.; O’Connor, N.E.; Regan, F. A neural network approach to smarter sensor networks for water quality monitoring. Sensors 2012, 12, 4605–4632. [Google Scholar] [CrossRef]
- Broring, A.; Maue, P.; Janowicz, K.; Nust, D.; Malewski, C. Semantically-enabled sensor plug & play for the sensor web. Sensors 2011, 11, 7568–7605. [Google Scholar] [CrossRef]
- Cabral, J.P. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, X.; Dong, T. A Microfluidic Device for Continuous Sensing of Systemic Acute Toxicants in Drinking Water. Int. J. Environ. Res. Public Health 2013, 10, 6748-6763. https://doi.org/10.3390/ijerph10126748
Zhao X, Dong T. A Microfluidic Device for Continuous Sensing of Systemic Acute Toxicants in Drinking Water. International Journal of Environmental Research and Public Health. 2013; 10(12):6748-6763. https://doi.org/10.3390/ijerph10126748
Chicago/Turabian StyleZhao, Xinyan, and Tao Dong. 2013. "A Microfluidic Device for Continuous Sensing of Systemic Acute Toxicants in Drinking Water" International Journal of Environmental Research and Public Health 10, no. 12: 6748-6763. https://doi.org/10.3390/ijerph10126748
APA StyleZhao, X., & Dong, T. (2013). A Microfluidic Device for Continuous Sensing of Systemic Acute Toxicants in Drinking Water. International Journal of Environmental Research and Public Health, 10(12), 6748-6763. https://doi.org/10.3390/ijerph10126748