Prevalence of Fetal Alcohol Syndrome and Maternal Characteristics in a Sample of Schoolchildren from a Rural Province of Croatia
Abstract
:1. Introduction
2. Experimental Section
3. Results
3.1. Maternal Characteristics
| Variable | Number | % |
|---|---|---|
| Education level | ||
| Elementary school | 30 | 3.3 |
| High school | 692 | 75.5 |
| College | 63 | 6.9 |
| University | 47 | 5.1 |
| Unanswered | 85 | 9.3 |
| Employment status | ||
| Unemployed | 253 | 27.6 |
| Employed | 660 | 72.0 |
| Unanswered | 4 | 0.4 |
| Marital status | ||
| Living with a partner | 872 | 95.1 |
| Not living with partner | 39 | 4.3 |
| Unanswered | 6 | 0.7 |
| Tobacco use | ||
| Current smokers | 188 | 20.5 |
| Pregnancy smokers | 91 | 9.9 |
| Current alcohol consumption | 145 | 15.8 |
| 1–3 drinks per week | 139 | 15.2 |
| 3–7 drinks per week | 6 | 0.7 |
| Current binge drinking | 53 | 5.7 |
| 1× in the last 3 months | 23 | 2.5 |
| 2–3× in the last 3 months | 24 | 2.6 |
| >4× in the last 3 months | 6 | 0.7 |
| Current alcohol consumption admitted | 167 | 18.2 |
| Weekly alcohol consumption | 114 | 12.4 |
| Weekly alcohol consumption and binge drinking | 31 | 3.4 |
| Binge drinking | 22 | 2.4 |
| Current alcohol consumption negative | 728 | 79.4 |
| Current alcohol consumption unanswered | 22 | 2.4 |
| Attitude towards alcohol effect on pregnancy outcome | ||
| Positive | 7 | 0.8 |
| Not answered | 26 | 2.8 |
| Negative | 884 | 96.4 |
| Pregnancy alcohol consumption | 37 | 4.0 |
| 1–3 drinks per week | 37 | 4.0 |
| 3–7 drinks per week | 0 | 0 |
| Pregnancy binge drinking | 13 | 1.4 |
| 1× during pregnancy | 2 | 0.2 |
| 2–3× during pregnancy | 11 | 1.2 |
| >4× during pregnancy | 0 | 0 |
| Type of alcohol consumed during pregnancy | ||
| Beer | 64 | 6.9 |
| Wine | 33 | 3.6 |
| Spirits | 1 | 0.1 |
| Beer + wine | 5 | 0.5 |
| Beer + spirits/wine + spirits/beer + wine + spirits | 0 | 0 |
| Trimester of alcohol consumption | ||
| 1st trimester | 26 | 2.8 |
| 2nd trimester | 7 | 0.8 |
| 3rd trimester | 12 | 1.3 |
| Entire pregnancy | 25 | 2.7 |
| Pregnancy alcohol consumption admitted (positive answer to one or more pregnancy alcohol consumption questions) | 105 | 11.5 |
| Pregnancy alcohol consumption negative (negative answer to all pregnancy alcohol consumption questions) | 795 | 86.7 |
| Pregnancy alcohol consumption unanswered (all pregnancy alcohol consumption questions unanswered) | 17 | 1.9 |
3.2. Characteristics of Examined Schoolchildren
| Variable | Number | % |
|---|---|---|
| Male | 423 | 51.3 |
| Female | 401 | 48.7 |
| Growth retardation (height and/or weight ≤10th centile) | 161 | 19.5 |
| Head circumference ≤10th centile | 79 | 9.6 |
| 2 FAS facial features | 172 | 20.9 |
| 3 FAS facial features | 77 | 9.3 |
| FAS | 14 | 1.7 |
| FAS with confirmed alcohol exposure | 4 | 0.5 |
| FAS with negative alcohol exposure | 10 | 1.2 |
| FAS with unanswered alcohol exposure | 0 | 0 |
| PFAS | 41 | 5.0 |
| PFAS with confirmed alcohol exposure | 6 | 0.7 |
| PFAS with negative alcohol exposure | 31 | 3.7 |
| PFAS with unanswered alcohol exposure | 4 | 0.5 |
3.3. FAS/PFAS Group of Children
| Maternal characteristics | Children without FAS/PFAS | Children with FAS/PFAS | p | ||
|---|---|---|---|---|---|
| N = 769 | N = 55 | ||||
| N | % | N | % | ||
| Maternal age | |||||
| <35 years | 421 | 54.7 | 32 | 58.2 | 0.855 (NS) |
| 35–45 years | 305 | 39.7 | 20 | 36.4 | |
| >45 years | 18 | 2.3 | 1 | 1.8 | |
| unanswered | 25 | 3.3 | 2 | 3.6 | |
| Education level | |||||
| elementary school | 27 | 3.5 | 0 | 0.0 | 0.089 (NS) |
| high school | 580 | 75.4 | 46 | 83.6 | |
| college | 56 | 7.3 | 0 | 0.0 | |
| university | 39 | 5.1 | 3 | 5.5 | |
| unanswered | 67 | 8.7 | 6 | 10.9 | |
| Employment status | |||||
| employed | 558 | 72.6 | 36 | 65.5 | 0.243 (NS) |
| unemployed | 209 | 27.2 | 19 | 34.5 | |
| unanswered | 2 | 0.3 | 0 | 0.0 | |
| Marital status | |||||
| living with partner | 731 | 95.1 | 53 | 96.4 | 0.778 (NS) |
| living without partner | 34 | 4.4 | 2 | 3.6 | |
| unanswered | 4 | 0.5 | 0 | 0.0 | |
| Current alcohol consumption | |||||
| admitted | 141 | 18.3 | 9 | 16.4 | >0.05 (NS) |
| negative | 614 | 79.8 | 42 | 76.3 | |
| unanswered | 14 | 1.8 | 4 | 7.2 | |
| Smoking during pregnancy | |||||
| admitted | 75 | 9.8 | 5 | 9.1 | 0.943 (NS) |
| negative | 681 | 88.6 | 47 | 85.5 | |
| unanswered | 13 | 1.7 | 3 | 5.5 | |
| Pregnancy alcohol consumption | |||||
| admitted | 80 | 10.4 | 10 | 18.2 | 0.001 |
| negative | 679 | 88.3 | 41 | 74.5 | |
| unanswered | 10 | 1.3 | 4 | 7.3 | |
| Alcohol consummation period | |||||
| 1st trimester | 21 | 2.7 | 0 | 0.0 | <0.001 |
| 2nd trimester | 6 | 0.8 | 0 | 0.0 | |
| 3rd trimester | 5 | 0.7 | 4 | 7.3 | |
| entire pregnancy | 19 | 2.5 | 3 | 5.5 | |
3.4. FAS/PFAS Prevalence Rate
4. Discussion
4.1. Participation Rate
4.2. Risk Factors for Pregnancy Alcohol Consumption
4.3. Alcohol Consumption during Pregnancy
4.4. Comparison of Maternal Characteristics between FAS/PFAS and Non-FAS/PFAS Group
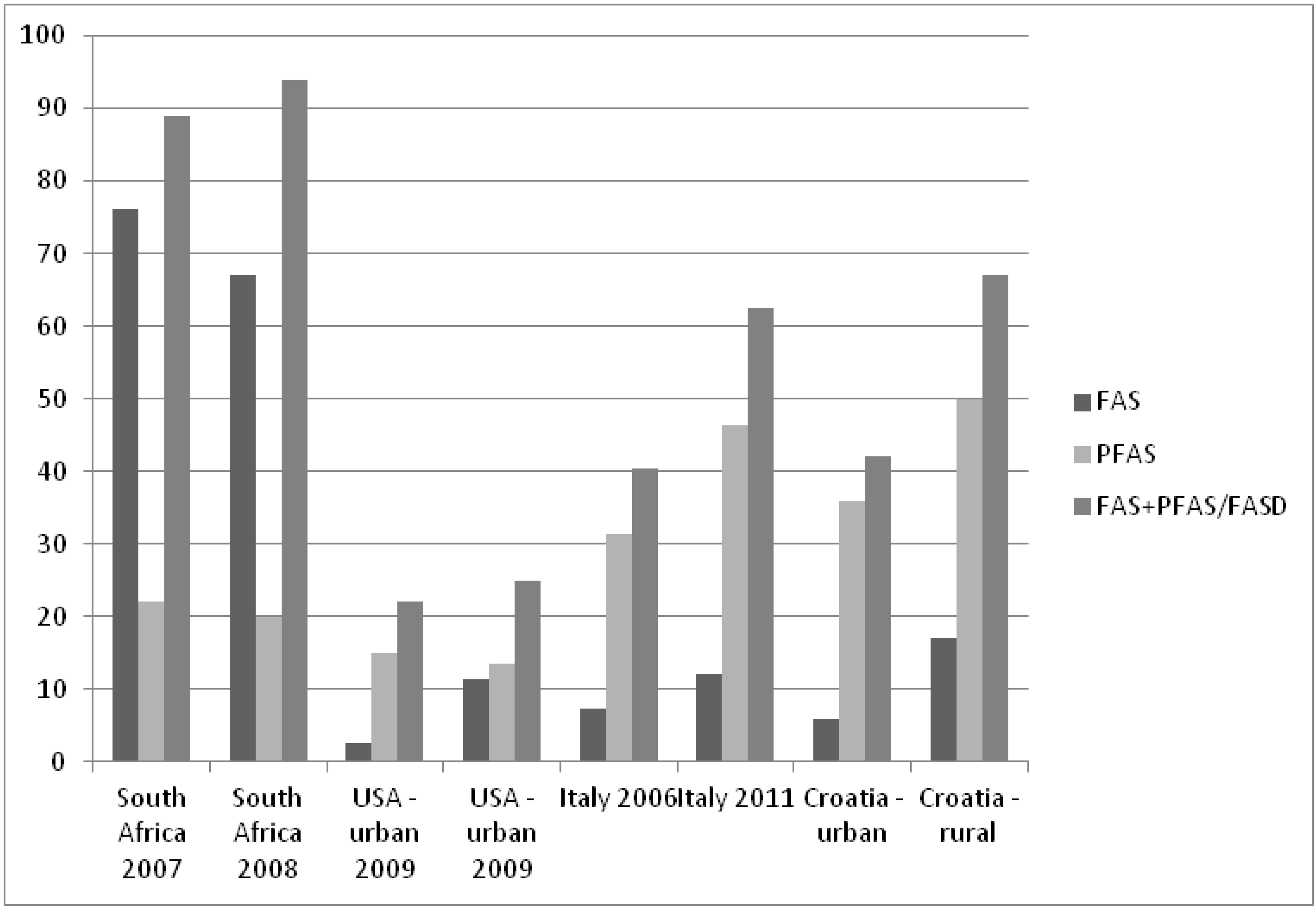
4.5. FAS/PFAS Prevalence Studies
4.6. Study Limitations
4.7. Future Studies
5. Conclusions
Acknowledgements
Conflict of interest
References
- Autti-Rämö, I.; Fagerlund, A.; Ervalahti, N.; Loimu, L.; Korkman, M.; Hoyme, H.E. Fetal alcohol spectrum disorders in Finland: Clinical delineation of 77 older children and adolescents. Am. J. Med. Genet. A 2006, 140, 137–143. [Google Scholar]
- Bruce, B.B.; Biousse, V.; Dean, A.L.; Newman, N.J. Neurologic and ophthalmic manifestations of fetal alcohol syndrome. Rev. Neurol. Dis. 2009, 6, 13–20. [Google Scholar]
- Manning, M.A.; Hoyme, H.E. Fetal alcohol spectrum disorders: A practical clinical approach to diagnosis. Neurosci. Biobehav. Rev. 2007, 31, 230–238. [Google Scholar] [CrossRef]
- Nayak, R.B.; Murthy, P. Fetal alcohol spectrum disorder. Indian Pediatr. 2008, 45, 977–983. [Google Scholar]
- Dalen, K.; Bruarøy, S.; Wentzel-Larsen, T.; Laegreid, L.M. Cognitive functioning in children prenatally exposed to alcohol and psychotropic drugs. Neuropediatrics 2009, 40, 162–167. [Google Scholar] [CrossRef]
- Mattson, S.N.; Schoenfeld, A.M.; Riley, E.P. Teratogenic effects of alcohol on brain and behavior. Alcohol Res. Health 2001, 25, 185–191. [Google Scholar]
- May, P.A.; Gossage, J.P. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res. Health 2001, 25, 159–167. [Google Scholar]
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef]
- Poitra, B.A.; Marion, S.; Dionne, M.; Wilkie, E.; Dauphinais, P.; Wilkie-Pepion, M.; Martsolf, J.T.; Klug, M.G.; Burd, L. A school-based screening program for fetal alcohol syndrome. Neurotoxicol. Teratol. 2003, 25, 725–729. [Google Scholar] [CrossRef]
- May, P.A.; Fiorentino, D.; Gossage, J.P.; Kalberg, W.O.; Hoyme, H.E.; Robinson, L.K.; Coriale, G.; Jones, K.L.; del Campo, M.; Tarani, L.; et al. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol Clin. Exp. Res. 2006, 30, 1562–1575. [Google Scholar] [CrossRef]
- May, P.A.; Fiorentino, D.; Coriale, G.; Kalberg, W.O.; Hoyme, H.E.; Aragón, A.S.; Buckley, D.; Stellavato, C.; Gossage, J.P.; Robinson, L.K.; et al. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: New estimated rates are higher than previous estimates. Int. J. Environ. Res. Public Health 2011, 8, 2331–2351. [Google Scholar] [CrossRef]
- Petković, G.; Barisić, I. FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod. Toxicol. 2010, 29, 237–241. [Google Scholar] [CrossRef]
- Bencevic-Striehl, H.; Malatestinic, D.; Vuletic, S. Regional differences in alcohol consumption in Croatia. Coll. Antropol. 2009, 33, 39–41. [Google Scholar]
- Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis; Center for Disease Control and Prevention: Atlanta, GA, USA, 2004; Available online: http://www.cdc.gov/ncbddd/fasd/documents/fas_guidelines_accessible.pdf (accessed on 19 February 2013).
- Kaskutas, L.A.; Graves, K. An alternative to standard drinks as a measure of alcohol consumption. J. Subst. Abuse 2000, 12, 67–78. [Google Scholar] [CrossRef]
- Clarren, S.K.; Randels, S.P.; Sanderson, M.; Fineman, R.M. Screening for fetal alcohol syndrome in primary schools: A feasibility study. Teratology 2001, 63, 3–10. [Google Scholar] [CrossRef]
- Astley, S.J.; Clarren, S.K. Measuring the facial phenotype of individuals with prenatal alcohol exposure: Correlations with brain dysfunction. Alcohol Alcohol. 2001, 36, 147–159. [Google Scholar]
- Juresa, V.; Musil, V.; Tiljak, M.K. Croatian Reference Values for Weight, Height, Body Mass Index for Boys and Girls 6.5 to 18.5 Years of Age; School of Public Health Andrija Štampar: Zagreb, Croatia, 2009; Available online: http://http://www.mef.hr/druga.php?grupa=020332050100 (accessed on 19 February 2013).
- Prader, A.; Largo, R.H.; Molinari, L.; Issler, C. Physical growth of Swiss children from birth to 20 years of age. Helv. Paediatr. Acta 1989, 52, 1–125. [Google Scholar]
- Thomas, I.T.; Gaitantzis, Y.A.; Frias, J.L. Palpebral fissure length from 29 weeks gestation to 14 years. J. Pediatr. 1987, 111, 267–268. [Google Scholar] [CrossRef]
- Hoyme, H.E.; May, P.A.; Kalberg, W.O.; Kodituwakku, P.; Gossage, J.P.; Trujillo, P.M.; Buckley, D.G.; Miller, J.H.; Aragon, A.S.; Khaole, N.; et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 institute of medicine criteria. Pediatrics 2005, 115, 39–47. [Google Scholar]
- Morojele, N.K.; London, L.; Olorunju, S.A.; Matjila, M.J.; Davids, A.S.; Rendall-Mkosi, K.M. Predictors of risk of alcohol-exposed pregnancies among women in an urban and a rural area of South Africa. Soc. Sci. Med. 2010, 70, 534–542. [Google Scholar] [CrossRef]
- Petković, G.; Barišić, I. Alcohol drinking and smoking habits during pregnancy in a sample of Croatian urban mothers. Paediatr. Croat. 2009, 2, 14. [Google Scholar]
- Samardžić, S.; Marvinac, G.V.; Prlić, A. Regional pattern of smoking in Croatia. Colleg. Antropolog. 2009, 33, 43–46. [Google Scholar]
- De Chazeron, I.; Llorca, P.M.; Ughetto, S.; Vendittelli, F.; Boussiron, D.; Sapin, V.; Coudore, F.; Lemery, D. Is pregnancy the time to change alcohol consumption habits in France? Alcohol Clin. Exp. Res. 2008, 32, 868–873. [Google Scholar]
- Donnelly, J.C.; Cooley, S.M.; Walsh, T.A.; Sarkar, R.; Durnea, U.; Geary, M.P. Illegal drug use, smoking and alcohol consumption in a low-risk Irish primigravid population. J. Perinat. Med. 2008, 36, 70–72. [Google Scholar]
- Fiorentino, D.; Coriale, G.; Spagnolo, P.A.; Prastaro, A.; Attilia, M.L.; Mancinelli, R.; Ceccanti, M. Fetal alcohol syndrome disorders: Experience on the field. The Lazio study preliminary report. Ann. Ist. Super Sanita. 2006, 42, 53–57. [Google Scholar]
- Floyd, R.L.; Sidhu, J.S. Monitoring prenatal alcohol exposure. Am. J. Med. Genet. C Semin. Med. Genet. 2004, 127, 3–9. [Google Scholar] [CrossRef]
- Sun, Y.; Strandberg-Larsen, K.; Vestergaard, M.; Christensen, J.; Nybo Andersen, A.M.; Grønbaek, M.; Olsen, J. Binge drinking during pregnancy and risk of seizures in childhood: A study based on the Danish National Birth Cohort. Am. J. Epidemiol. 2009, 169, 313–322. [Google Scholar]
- Tough, S.; Tofflemire, K.; Clarke, M.; Newburn-Cook, C. Do women change their drinking behaviors while trying to conceive? An opportunity for preconception counseling. Clin Med. Res. 2006, 4, 97–105. [Google Scholar] [CrossRef]
- Alcohol Use among Pregnant and Nonpregnant Woman of Childbearing Age, United States, 1991–2005; Center for Disease Control and Prevention: Atlanta, GA, USA, 2009; Available online: http://www.cdc.gov/ncbddd/fasd/articles.html (accessed on 19 February 2013).
- May, P.A.; Brooke, L.; Gossage, J.P.; Croxford, J.; Adnams, C.; Jones, K.L.; Robinson, L.; Viljoen, D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am. J. Public Health 2000, 90, 1905–1912. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Marais, A.S.; Adnams, C.M.; Hoyme, H.E.; Jones, K.L.; Robinson, L.K.; Khaole, N.C.; Snell, C.; Kalberg, W.O.; et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007, 88, 259–271. [Google Scholar] [CrossRef]
- Urban, M.; Chersich, M.F.; Fourie, L.A.; Chetty, C.; Olivier, L.; Viljoen, D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: Prevalence and risk factors. S Afr. Med. J. 2008, 98, 877–882. [Google Scholar]
- Viljoen, D.L.; Gossage, J.P.; Brooke, L.; Adnams, C.M.; Jones, K.L.; Robinson, L.K.; Hoyme, H.E.; Snell, C.; Khaole, N.C.; Kodituwakku, P.; et al. Fetal alcohol syndrome epidemiology in a South African community: A second study of a very high prevalence area. J. Stud. Alcohol 2005, 66, 593–604. [Google Scholar]
- Petković, G.; Barišić, I. Fetal alcohol syndrome in urban schools. In Pregnancy and Alcohol Consumption; Hoffman, J.D., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 281–298. [Google Scholar]
- Khaole, N.C.; Ramchandani, V.A.; Viljoen, D.L.; Li, T.K. A pilot study of alcohol exposure and pharmacokinetics in women with or without children with fetal alcohol syndrome. Alcohol Alcohol. 2004, 39, 503–508. [Google Scholar] [CrossRef]
- McCarver, D.G. ADH2 and CYP2E1 genetic polymorphisms: Risk factors for alcohol-related birth defects. Drug Metab. Dispos. 2001, 29, 562–565. [Google Scholar]
- Ramchandani, V.A.; Kwo, P.Y.; Li, T.K. Effect of food and food composition on alcohol elimination rates in healthy men and women. J. Clin. Pharmacol. 2001, 41, 1345–1350. [Google Scholar] [CrossRef]
- Pichini, S.; Marchei, E.; Vagnarelli, F.; Tarani, L.; Raimondi, F.; Maffucci, R.; Sacher, B.; Bisceglia, M.; Rapisardi, G.; Elicio, M.R.; et al. Assessment of prenatal exposure to ethanol by meconium analysis: Results of an Italian multicentre study. Alcohol Clin. Exp. Res. 2012, 36, 417–424. [Google Scholar]
- Joya, X.; Friguls, B.; Ortigosa, S.; Papaseit, E.; Martínez, S.E.; Manich, A.; Garcia-Algar, O.; Pacifici, R.; Vall, O.; Pichini, S. Determination of maternal-fetal biomarkers of prenatal exposure to ethanol: A review. J. Pharm. Biomed. Anal. 2012, 69, 209–222. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Petković, G.; Barišić, I. Prevalence of Fetal Alcohol Syndrome and Maternal Characteristics in a Sample of Schoolchildren from a Rural Province of Croatia. Int. J. Environ. Res. Public Health 2013, 10, 1547-1561. https://doi.org/10.3390/ijerph10041547
Petković G, Barišić I. Prevalence of Fetal Alcohol Syndrome and Maternal Characteristics in a Sample of Schoolchildren from a Rural Province of Croatia. International Journal of Environmental Research and Public Health. 2013; 10(4):1547-1561. https://doi.org/10.3390/ijerph10041547
Chicago/Turabian StylePetković, Giorgie, and Ingeborg Barišić. 2013. "Prevalence of Fetal Alcohol Syndrome and Maternal Characteristics in a Sample of Schoolchildren from a Rural Province of Croatia" International Journal of Environmental Research and Public Health 10, no. 4: 1547-1561. https://doi.org/10.3390/ijerph10041547




