Socioeconomic Status Accounts for Rapidly Increasing Geographic Variation in the Incidence of Poor Fetal Growth
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Source
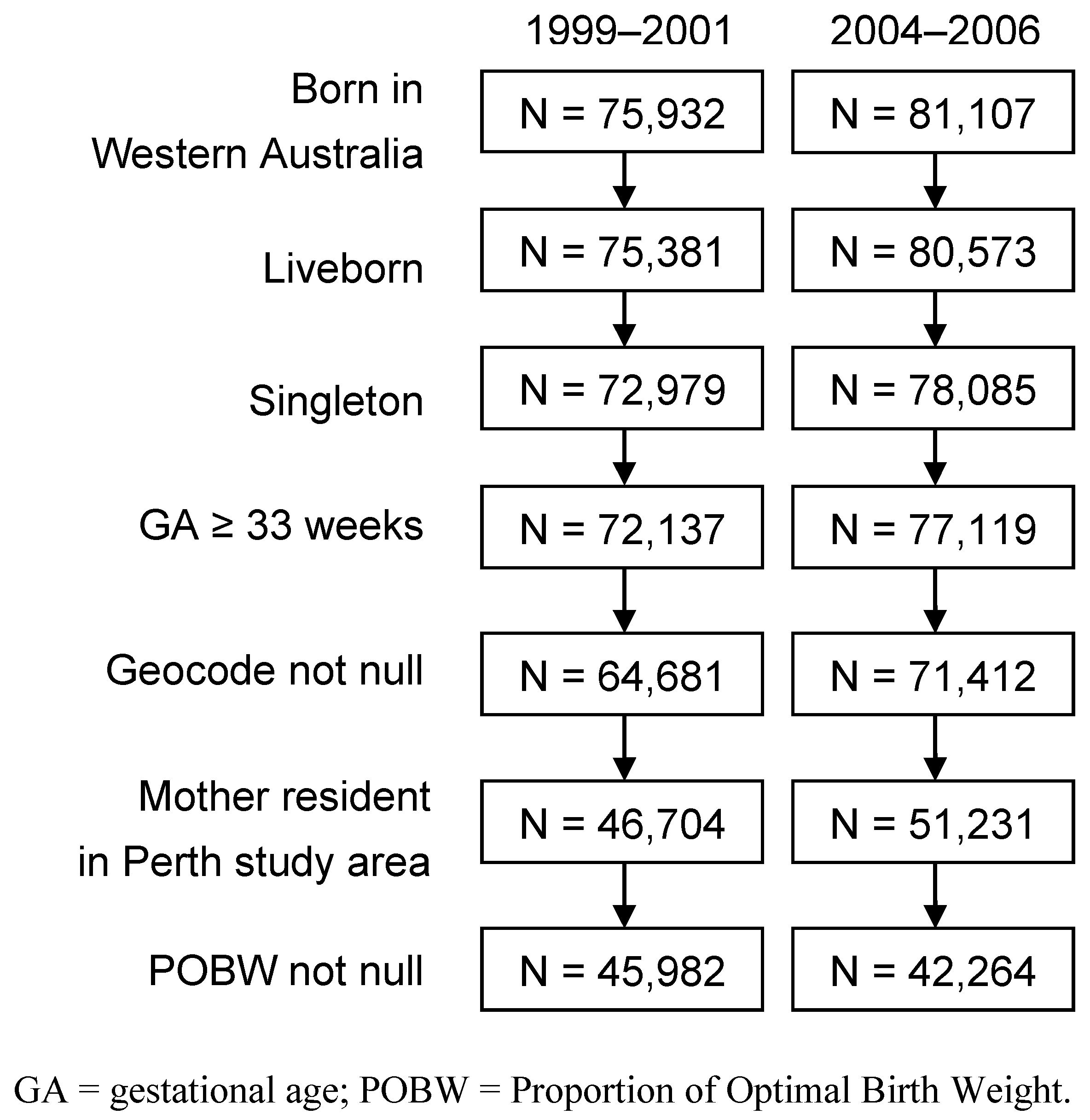
2.3. Spatial Units
2.4. Definition of Poor Fetal Growth
2.5. Socioeconomic Status
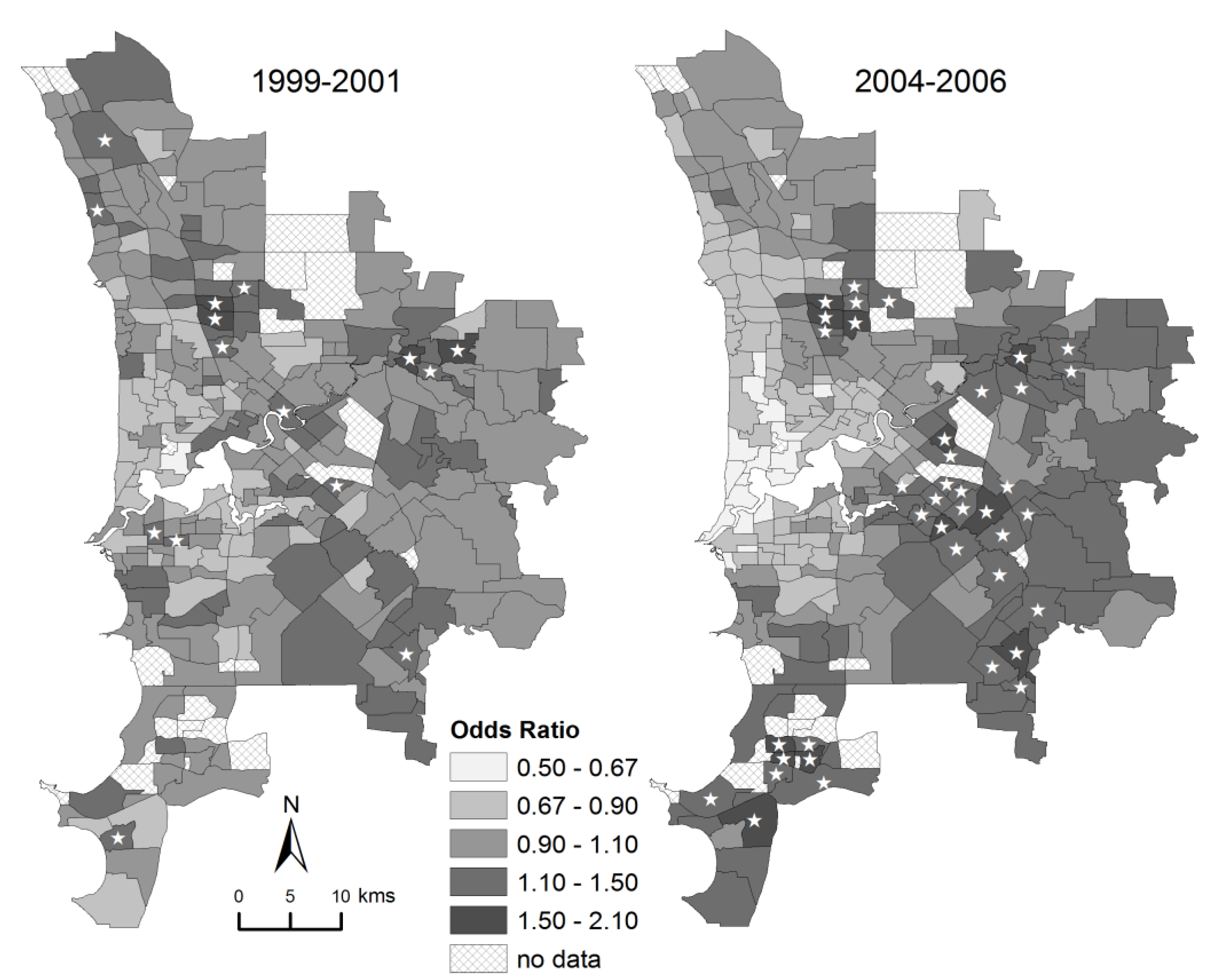
2.6. Models of Poor Fetal Growth

2.7. Additional Software
3. Results
| Period | Model | Effect(s) | IQOR a |
|---|---|---|---|
| 1999–2001 | 2. Random effects | Spatially-uncorrelated random effect | 1.13 |
| Spatially-correlated random effect | 1.06 | ||
| Combined random effects | 1.20 | ||
| 4. Full model | Spatially-uncorrelated random effect | 1.07 | |
| Spatially-correlated random effect | 1.03 | ||
| Combined random effects | 1.09 | ||
| Socioeconomic status | 1.41 | ||
| 2004–2006 | 2. Random effects | Spatially-uncorrelated random effect | 1.03 |
| Spatially-correlated random effect | 1.40 | ||
| Combined random effects | 1.40 | ||
| 4. Full model | Spatially-uncorrelated random effect | 1.04 | |
| Spatially-correlated random effect | 1.06 | ||
| Combined random effects | 1.09 | ||
| Socioeconomic status | 1.46 |
| Period | Model | DIC | pD | p | β1 |
|---|---|---|---|---|---|
| 1999−2001 | 1. Null model | 1,277.3 | 1.0 | 0.050 (0.048, 0.052) | |
| 2. Random effects | 1,217.3 | 81.2 | 0.047 (0.045, 0.050) | ||
| 3. Fixed effect | 1,195.4 | 2.0 | 0.050 (0.030, 0.087) | −0.21 (−0.25, −0.16) | |
| 4. Full model | 1,180.5 | 48.7 | 0.049 (0.025, 0.098) | −0.22 (−0.28, −0.17) | |
| 2004−2006 | 1. Null model | 1,320.3 | 1.0 | 0.049 (0.047, 0.052) | |
| 2. Random effects | 1,202.8 | 72.7 | 0.047 (0.045, 0.050) | ||
| 3. Fixed effect | 1,183.3 | 2.0 | 0.052 (0.028, 0.097) | −0.27 (−0.32, −0.23) | |
| 4. Full model | 1,176.5 | 38.0 | 0.052 (0.022, 0.111) | −0.26 (−0.31, −0.19) |
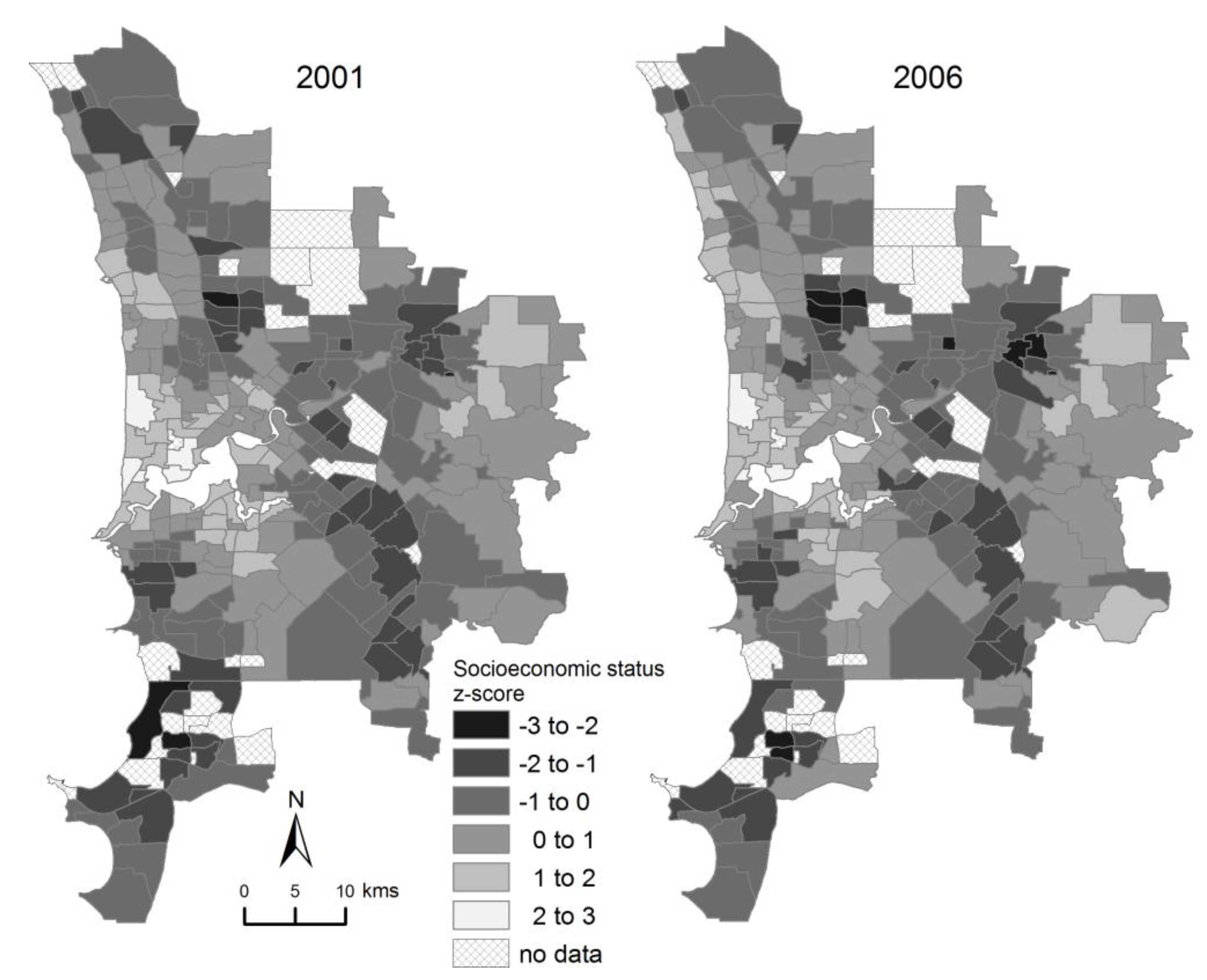
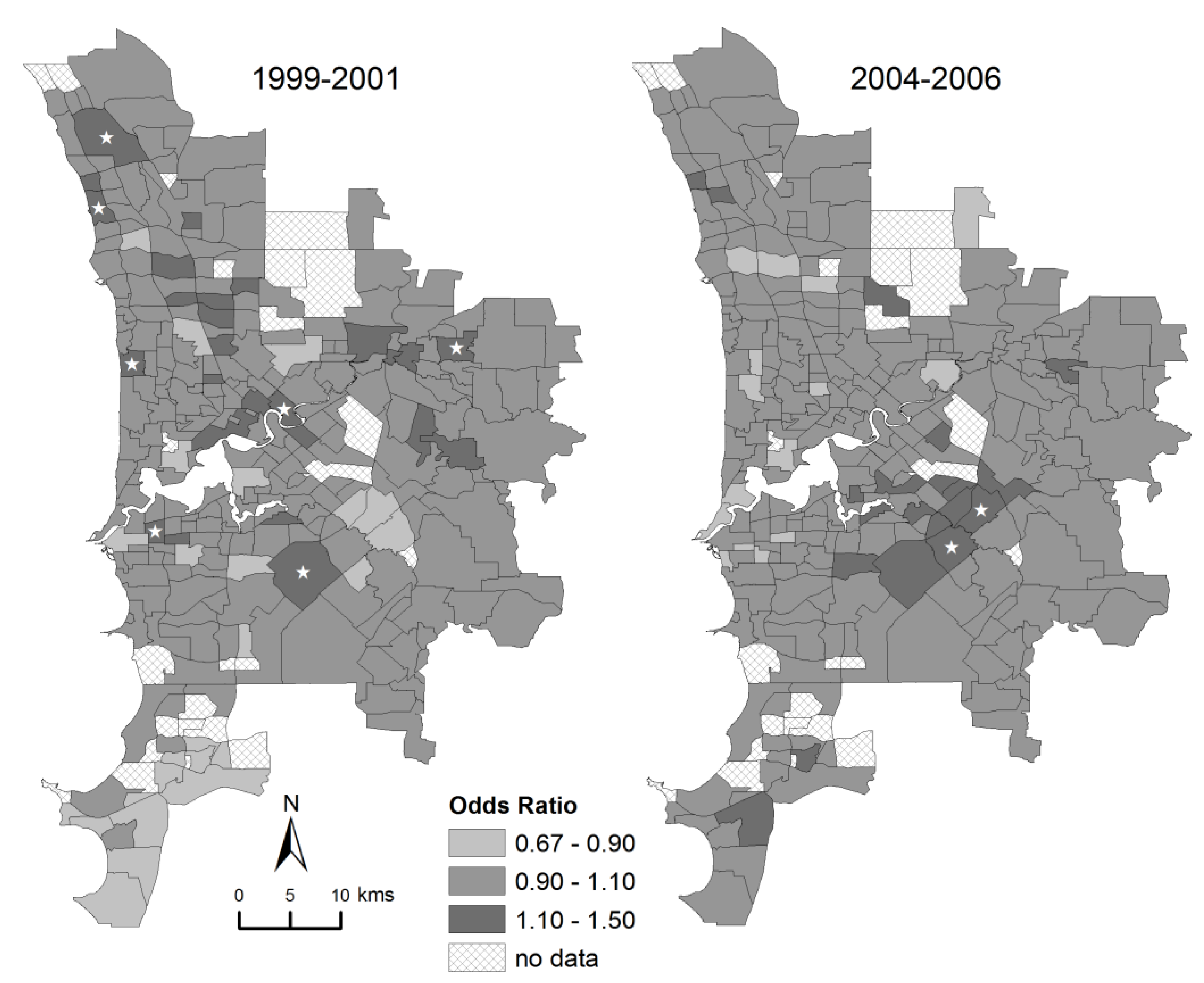
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Giapros, V.; Drougia, A.; Krallis, N.; Theocharis, P.; Andronikou, S. Morbidity and mortality patterns in small-for-gestational age infants born preterm. J. Matern. Fetal. Neonatal. Med. 2012, 25, 153–157. [Google Scholar]
- Grisaru-Granovsky, S.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Hammerman, C.; Samueloff, A.; Schimmel, M.S.; Israel Neonatal Network. Mortality and morbidity in preterm small-for-gestational-age infants: A population-based study. Am. J. Obstet. Gynecol. 2012, 206, 150. [Google Scholar] [CrossRef]
- Montes-Nunez, S.; Chavez-Corral, D.V.; Reza-Lopez, S.; Sanin, L.H.; Acosta-Maldonado, B.; Levario-Carrillo, M. Birth weight in children with birth defects. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 102–107. [Google Scholar] [CrossRef]
- Barker, D.J.P. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006, 49, 270–283. [Google Scholar] [CrossRef]
- Kramer, M.S.; Seguin, L.; Lydon, J.; Goulet, L. Socio-economic disparities in pregnancy outcome: Why do the poor fare so poorly? Paediatr. Perinat. Epidemiol. 2000, 14, 194–210. [Google Scholar] [CrossRef]
- Arif, M.A.; Qureshi, A.H.; Jafarey, S.N.; Alam, S.E.; Arif, K. Maternal sociocultural status: A novel assessment of risk for the birth of small for gestational age, low birth weight infants. J. Obstet. Gynaecol. Res. 1998, 24, 215–222. [Google Scholar] [CrossRef]
- Alexander, G.R.; Kogan, M.; Martin, J.; Papiernik, E. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin. Obstet. Gynecol. 1998, 41, 115–125. [Google Scholar] [CrossRef]
- Beard, J.R.; Lincoln, D.; Donoghue, D.; Taylor, D.; Summerhayes, R.; Dunn, T.M.; Earnest, A.; Morgan, G. Socioeconomic and maternal determinants of small-for-gestational age births: Patterns of increasing disparity. Acta Obstet. Gynecol. Scand. 2009, 88, 575–583. [Google Scholar]
- Windham, G.C.; Fenster, L.; Hopkins, B.; Swan, S.H. The association of moderate maternal and paternal alcohol consumption with birthweight and gestational age. Epidemiology 1995, 6, 591–597. [Google Scholar]
- Lang, J.M.; Lieberman, E.; Cohen, A. A comparison of risk factors for preterm labor and term small-for-gestational-age birth. Epidemiology 1996, 7, 369–376. [Google Scholar]
- Voskamp, B.J.; Kazemier, B.M.; Ravelli, A.C.J.; Schaaf, J.; Mol, B.W.J.; Pajkrt, E. Recurrence of small-for-gestational-age pregnancy: Analysis of first and subsequent singleton pregnancies in The Netherlands. Am. J. Obstet. Gynecol. 2013, 208, 374. [Google Scholar] [CrossRef]
- Love, C.; David, R.J.; Rankin, K.M.; Collins, J.W., Jr. Exploring weathering: effects of lifelong economic environment and maternal age on low birth weight, small for gestational age, and preterm birth in African-American and white women. Obstet. Gynecol. Surv. 2010, 65, 685–686. [Google Scholar]
- Godfrey, K.; Robinson, S.; Barker, D.J.P.; Osmond, C.; Cox, V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. Br. Med. J. 1996, 312, 410–414. [Google Scholar] [CrossRef]
- Farley, T.A.; Mason, K.; Rice, J.; Habel, J.D.; Scribner, R.; Cohen, D.A. The relationship between the neighbourhood environment and adverse birth outcomes. Paediatr. Perinat. Epidemiol. 2006, 20, 188–200. [Google Scholar]
- Pereira, G.; Nassar, N.; Cook, A.; Bower, C. Traffic emissions are associated with reduced fetal growth in areas of Perth, Western Australia: An application of the AusRoads dispersion model. Aust. N. Z. J. Public Health 2011, 35, 451–458. [Google Scholar] [CrossRef]
- Bove, F.; Shim, Y.; Zeitz, P. Drinking water contaminants and adverse pregnancy outcomes: A review. Environ. Health Perspect. 2002, 110, 61–74. [Google Scholar]
- Sebayang, S.K.; Dibley, M.J.; Kelly, P.J.; Shankar, A.V.; Shankar, A.H.; SUMMIT Study Group. Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: Analyses of the birthweight cohort of the SUMMIT trial. Trop. Med. Int. Health 2012, 17, 938–950. [Google Scholar] [CrossRef]
- South, A.P.; Jones, D.E.; Hall, E.S.; Huo, S.; Meinzen-Derr, J.; Liu, L.; Greenberg, J.M. Spatial analysis of preterm birth demonstrates opportunities for targeted intervention. Matern. Child Health J. 2012, 16, 470–478. [Google Scholar] [CrossRef]
- Braveman, P.A.; Cubbin, C.; Egerter, S.; Chideya, S.; Marchi, K.S.; Metzler, M.; Posner, S. Socioeconomic status in health research—One size does not fit all. JAMA 2005, 294, 2879–2888. [Google Scholar] [CrossRef]
- Pickett, K.E.; Pearl, M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: A critical review. J. Epidemiol. Community Health 2001, 55, 111–122. [Google Scholar]
- Langridge, A.T.; Li, J.; Nassar, N.; Stanley, F.J. Community-level socioeconomic inequalities in infants with poor fetal growth in Western Australia, 1984 to 2006. Ann. Epidemiol. 2011, 21, 473–480. [Google Scholar] [CrossRef]
- Read, A.W.; Stanley, F.J. Small-for-gestational-age term birth: The contribution of socio-economic, behavioural and biological factors to recurrence. Paediatr. Perinat. Epidemiol. 1993, 7, 177–194. [Google Scholar] [CrossRef]
- Rip, M.R.; Keen, C.S.; Woods, D.L. Spatial variations of low-birth-weight in Cape-Town. J. Trop. Pediatr. 1987, 33, 333–336. [Google Scholar] [CrossRef]
- Pink, B. 2039.0 Information Paper: An Introduction to Socio-Economic Indexes for Areas (SEIFA), 2006; Australian Bureau of Statistics: Canberra, Australia, 2008.
- Trewin, D. 2039.0 Information Paper. Census of Population and Housing Socio-Economic Indexes for Areas, Australia, 2001; Australian Bureau of Statistics: Canberra, Austraila, 2003.
- Boyd, H.A.; Flanders, W.D.; Addiss, D.G.; Waller, L.A. Residual spatial correlation between geographically referenced observations—A Bayesian hierarchical modeling approach. Epidemiology 2005, 16, 532–541. [Google Scholar] [CrossRef]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Sebert Kuhlmann, A.K.; Brett, J.; Thomas, D.; Sain, S.R. Environmental characteristics associated with pedestrian-motor vehicle collisions in Denver, Colorado. Am. J. Public Health 2009, 99, 1632–1637. [Google Scholar] [CrossRef]
- Dominguez-Berjon, M.F.; Gandarillas, A.; Segura del Pozo, J.; Zorrilla, B.; Soto, M.J.; Lopez, L.; Duque, I.; Marta, M.I.; Abad, I. Census tract socioeconomic and physical environment and cardiovascular mortality in the Region of Madrid (Spain). J. Epidemiol. Community Health 2010, 64, 1086–1093. [Google Scholar] [CrossRef]
- Lorenzo-Luaces Alvarez, P.; Guerra-Yi, M.E.; Faes, C.; Galan Alvarez, Y.; Molenberghs, G. Spatial analysis of breast and cervical cancer incidence in small geographical areas in Cuba, 1999–2003. Eur. J. Cancer Prev. 2009, 18, 395–403. [Google Scholar] [CrossRef]
- Clements, A.C.A.; Barnett, A.G.; Cheng, Z.W.; Snow, R.W.; Zhou, H.N. Space-time variation of malaria incidence in Yunnan province, China. Malar. J. 2009, 8, 180. [Google Scholar] [CrossRef]
- Kazembe, L.N.; Namangale, J.J. A Bayesian multinomial model to analyse spatial patterns of childhood co-morbidity in Malawi. Eur. J. Epidemiol. 2007, 22, 545–556. [Google Scholar] [CrossRef]
- Lawson, A.B.; Browne, W.B.; Vidal-Rodeiro, C.L. Disease Mapping with WinBUGS and MLwiN; John Wiley and Sons: Chichester, UK, 2003. [Google Scholar]
- Mueller, I.; Vounatsou, P.; Allen, B.J.; Smith, T. Spatial patterns of child growth in Papua New Guinea and their relation to environment, diet, socio-economic status and subsistence activities. Ann. Hum. Biol. 2001, 28, 263–280. [Google Scholar] [CrossRef]
- Pattenden, S.; Casson, K.; Cook, S.; Dolk, H. Geographical variation in infant mortality, stillbirth and low birth weight in Northern Ireland, 1992–2002. J. Epidemiol. Community Health 2011, 65, 1159–1165. [Google Scholar] [CrossRef]
- Congdon, P. Assessing the impact of socioeconomic variables on small area variations in suicide outcomes in England. Int. J. Environ. Res. Public Health 2013, 10, 158–177. [Google Scholar] [CrossRef]
- Stanley, F.J.; Croft, M.L.; Gibbins, J.; Read, A.W. A population database for maternal and child health research in Western Australia using record linkage. Paediatr. Perinat. Epidemiol. 1994, 8, 433–447. [Google Scholar] [CrossRef]
- Blair, E.M.; Liu, Y.; de Klerk, N.H.; Lawrence, D.M. Optimal fetal growth for the Caucasian singleton and assessment of appropriateness of fetal growth: An analysis of a total population perinatal database. BMC Pediatr. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Pink, B. Census Geographic Areas, Australia, 2006. In Statistical Geography; Australian Bureau of Statistics: Canberra, Australia, 2007; Volume 2. [Google Scholar]
- Dobbins, T.A.; Sullivan, E.A.; Roberts, C.L.; Simpson, J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med. J. Aust. 2012, 197, 291–294. [Google Scholar] [CrossRef]
- Besag, J.; York, J.; Mollie, A. Bayesian image-restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Best, N.; Richardson, S.; Thomson, A. A comparison of Bayesian spatial models for disease mapping. Stat. Methods Med. Res. 2005, 14, 35–59. [Google Scholar] [CrossRef]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Gelman, A. Prior distributions for variance parameters in hierarchical models. Bayesian Anal. 2006, 1, 515–533. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.R.; van der Linde, A. Bayesian measures of model complexity and fit. J. Roy. Statist. Soc. Ser. B 2002, 64, 583–616. [Google Scholar] [CrossRef]
- Geweke, J. Evaluating the Accuracy of Sampling-Based Approaches to the Calculation of Posterior Moments. In Bayesian Statistics 4; Bernardo, J.M., Berger, J.O., Dawid, A.P., Smith, A.F.M., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 169–193. [Google Scholar]
- Oden, N. Adjusting Morans-I for population-density. Stat. Med. 1995, 14, 17–26. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar]
- Kennewell, C.; Shaw, B.J. Perth, Western Australia. Cities 2008, 25, 243–255. [Google Scholar] [CrossRef]
- Morrison, J.; Najman, J.M.; Williams, G.M.; Keeping, J.D.; Andersen, M.J. Socio-economic status and pregnancy outcome. An Australian study. Br. J. Obstet. Gynaecol. 1989, 96, 298–307. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ball, S.J.; Jacoby, P.; Zubrick, S.R. Socioeconomic Status Accounts for Rapidly Increasing Geographic Variation in the Incidence of Poor Fetal Growth. Int. J. Environ. Res. Public Health 2013, 10, 2606-2620. https://doi.org/10.3390/ijerph10072606
Ball SJ, Jacoby P, Zubrick SR. Socioeconomic Status Accounts for Rapidly Increasing Geographic Variation in the Incidence of Poor Fetal Growth. International Journal of Environmental Research and Public Health. 2013; 10(7):2606-2620. https://doi.org/10.3390/ijerph10072606
Chicago/Turabian StyleBall, Stephen J., Peter Jacoby, and Stephen R. Zubrick. 2013. "Socioeconomic Status Accounts for Rapidly Increasing Geographic Variation in the Incidence of Poor Fetal Growth" International Journal of Environmental Research and Public Health 10, no. 7: 2606-2620. https://doi.org/10.3390/ijerph10072606
APA StyleBall, S. J., Jacoby, P., & Zubrick, S. R. (2013). Socioeconomic Status Accounts for Rapidly Increasing Geographic Variation in the Incidence of Poor Fetal Growth. International Journal of Environmental Research and Public Health, 10(7), 2606-2620. https://doi.org/10.3390/ijerph10072606




