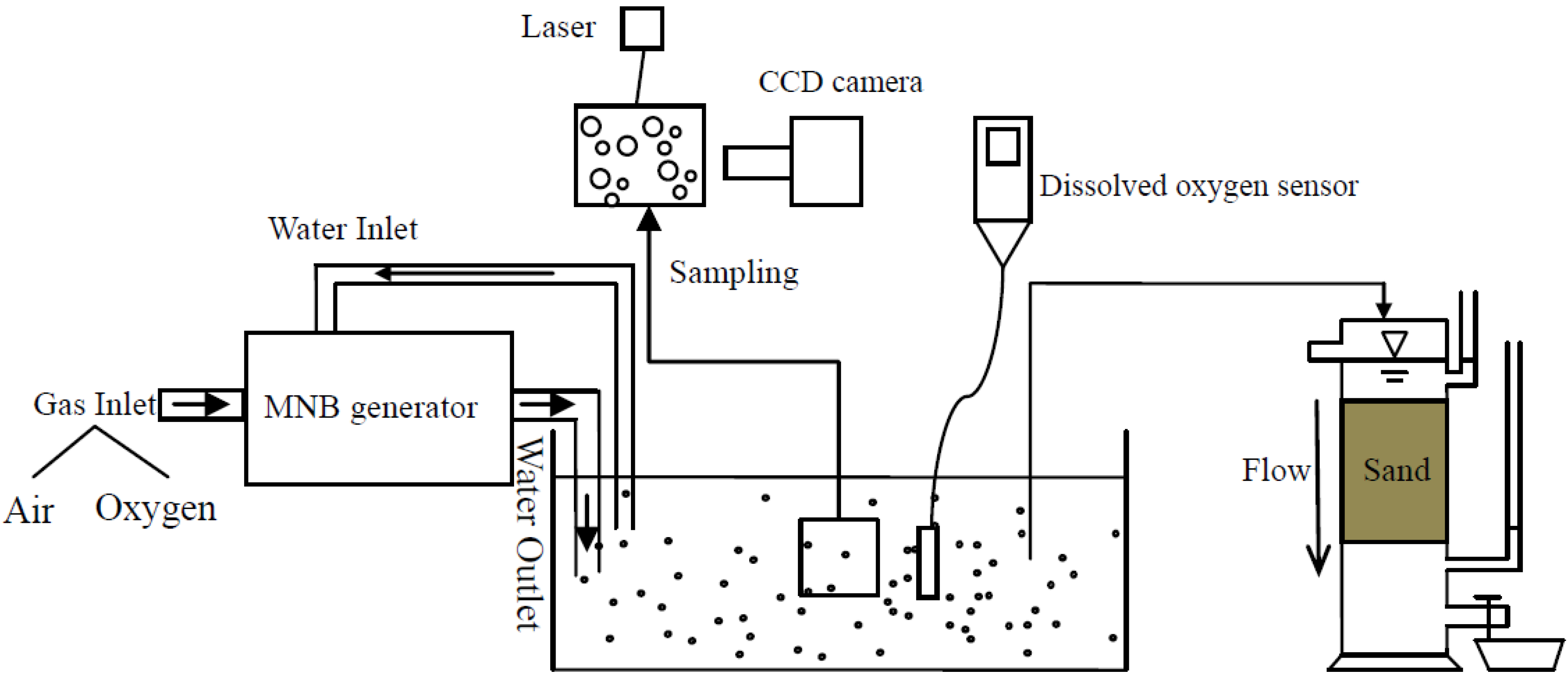
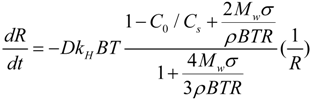3.3. The Oxygen Transfer Efficiency
As mentioned above, the spiral liquid flow type MNBs generator applied in this study had better oxygen transfer performance than other types of generators. The DO amount of the first four groups, in the form of percentage over saturation value (9.08 mg/L), changed with time, as shown in
Figure 4.
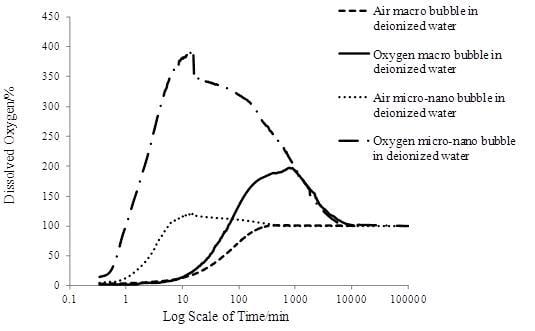
Figure 4 shows that MNBs accelerated the oxygen transfer process. For the same gas type, both the increasing DO concentration rate and the peak value of the MNBs were much higher than that of macrobubbles. For the same bubble size, oxygen enhanced the transfer process more than air. According to Fick’s law of diffusion [
32], the oxygen transfer efficiency is determined by the oxygen gradient from the inner bubble to the outer liquid. For oxygen MNBs, the oxygen concentration inside the bubble is higher than that inside the air MNBs, and much higher than that inside the air macro bubbles, according to Henry’s law [
33] that the gas concentration in liquid is proportional to the gas partial pressure. As shown in
Figure 4 the oxygen macrobubbles reached a higher DO peak value than that of air MNBs, it could be seen that a decrease in the bubble size made the oxygen transfer process faster, while the DO peak value was mainly determined by the gas type.
Figure 4.
Changing DO pattern with time.
Figure 4.
Changing DO pattern with time.
For the air macrobubbles in deionized water test, DO gradually increased with time and finally reached 100%, then remained constant. However, for the tests of MNBs in deionized water, DO increased rapidly and then reached a peak value, which was different for each group, and after that DO went down until it reached 100%. In detail, DO increased rapidly due to the large number of MNBs generated to be dissolved in water. It was nearly a linear increase as shown in
Figure 4. The average initial DO increase rate (AIDOIR) was determined, which indicated the mass transfer rate of the MNBs. The AIDOIR relates to the generator capacity of how many, how fast and how tiny bubbles can be generated. The water volume and gas type is associated with AIDOIR as well. Based on Epstein-Plesset model for bubble dissolution in aqueous media, the bubble radius
R can be written as follows [
34]:
where
t is time,
D is the diffusion coefficient of the gas in the medium,
kH is the Henry’s law constant,
B is the gas constant,
T is temperature,
Mw is the gas molecular weight, σ is surface tension, ρ is the density of gas in the bubble,
C0 is initial gas concentration in solution,
Cs is initial gas concentration in bubble. In this study,
C0 is assumed to be 0.
For a single bubble, the mass transfer rate
dm/dt is:
For test Group 3, an average bubble diameter of 33.4 μm was used and the bubble gas density ρ was calculated using Young-Laplace equation [
29,
30] and the ideal gas law [
35].
The internal pressure of MNBs is calculated from the Young-Laplace equation [
29,
30]:
where
Pin is the bubble internal pressure,
Pout is the liquid pressure outside the bubble.
Using the ideal gas law [
35] we have:
The density of the gas (ρ) inside bubbles is calculated from Equations (4) and (5):
Therefore, for a 33.44 μm-diameter air bubble, the gas density is 1.32 kg/m3.
Other parameter values are shown in
Table 5 [
36].
Table 5.
Simulation parameters.
Table 5.
Simulation parameters.
| Parameter | Value | Unit |
|---|
| D | 1.75 × 10−9 | m2/s |
| kH | 7.44 × 10−6 | mol/N·m |
| B | 8.3144 | J/mol·K |
| T | 293.15 | K |
| Mw | 29 | g/mol |
| σ | 0.072 | N/m |
Therefore, the air mass transfer rate for a single bubble is −5.41 × 10−7 mg/min. The bubble number is calculated by the bubble number counter system. The MNB water is placed in the transparent model box with the MNB water irradiated with a laser light, and CCD camera with a microscope can detect the scattered light from the bubbles. In this way, the MNBs can be imaged and recorded in the computer. The bubble number in a certain volume (calculated by the area and depth of image field) is counted. The air MNBs suspension observed is around 7.46 × 107 bubbles in 11 L water. Considering that 21% (volume) of air made up by oxygen, the oxygen mass transfer rate is −9.32 mg/min. Experimentally, the AIDOIR is 0.94 mg/(L·min), so in 11 L water, the total oxygen mass transfer rate is 10.38 mg/min. The simulation result is 10.2% smaller than the experimental results, which proves the experiment is convincing.
AIDOIR relates to the bubble size. Theoretically, an air bubble with a 1 mm diameter weighs approximately eight hundred thousand times the mass of an air bubble with a 10 μm diameter based on the Young-Laplace equation [
29,
30] and ideal gas law [
35]. Similar calculation as above can be carried out for the mass transfer rates of one 1 mm-diameter air bubble and eight hundred thousand 10 μm-diameter air bubbles.
For one 1 mm-diameter air bubble, the mass transfer rate is −3.38 × 10
−6 mg/min, while for eight hundred thousand 10 μm-diameter air bubbles, the mass transfer rate is −3.68 × 10
−2 mg/min. Therefore, ten thousand more mass transfer rate will be achieved if one 1 mm-diameter air bubble is divided to eight hundred thousand 10 μm-diameter air bubbles. That explains why the AIDOIR of MNBs is much higher than the macro bubbles in
Table 6.
Table 6.
AIDOIR 1, DOPV 2 and ST 3 of six groups.
Table 6.
AIDOIR 1, DOPV 2 and ST 3 of six groups.
| | AIDOIR (10−2 mg/L/min) | DOPV (mg/L) | ST (103 min) |
|---|
| Group 1 1 | 2.70 ± 0.20 | 9.89 ± 0.08 | - |
| Group 2 4 | 3.50 ± 0.30 | 19.1 ± 1.2 | 9.30 ± 0.12 |
| Group 3 4 | 94.4 ± 2.1 | 10.4 ± 0.3 | 0.58 ± 0.06 |
| Group 4 4 | 342 ± 19 | 34.2 ± 1.9 | 9.98 ± 0.14 |
| Group 5 4 | 74.1 ± 0.5 | 9.63 ± 0.07 | 1.86 ± 0.08 |
| Group 6 4 | 63.8 ± 0.3 | 9.57 ± 0.05 | 2.58 ± 0.09 |
As DO concentration increased, the DO concentration gradient between the water and the surrounding environment increased resulting in the DO diffusion from water to the atmosphere. The diffusion rate was proportional to the concentration gradient according to the Fick’s law. At a certain time, the rate of increasing DO concentration was equal to the diffusion rate, and the DO kept stable. In the tests of this study, the time was 15 min, which depended mainly on the generator capacity and water volume. When DO was stable, the DO value was defined as the DO peak value (DOPV). DOPV is the integral result of AIDOIR over a certain time (15 min). Therefore, it is determined by AIDOIR.
When DOPV was reached, the generator was stopped, so the DO is decreasing due to the DO diffusion from the water to the atmosphere. The time when DO decreased from DOPV to a stable value (100%) was defined as stagnation time (ST). ST indicated the life span of MNBs (the time all MNBs dissolve into water) and the diffusion rate. It was associated with the MNB properties: size, number, interface properties and gas type. Water volume and diffusion rate also influenced ST.
The diffusion rate was related to the DOPV. Higher DOPV led to higher diffusion rate. However, more DO diffused for higher DOPV. Therefore, ST was a complicated parameter which is needed further study. In this study, ST was a parameter to roughly show the durability of DO increasing effect.
Those three parameters (AIDOIR, DOPV and ST) of all six groups were listed in
Table 6. The AIDOIR of the oxygen micro-nanobubble group shown in
Table 6 was nearly 125 times faster than the air macrobubble group and the DOPV was nearly three times larger. Moreover, the air micro-nanobubble group (Group 3) achieved about 35 times the AIDOIR and 1.05 times the DOPV of the air macrobubble group (Group 1). Obvious enhancement in oxygen transfer efficiency could be seen from the comparison. With the surfactant added into the water, as shown in Groups 5 and 6, the AIDOIR reduced and was inversely proportional to the surfactant concentration compared to Group 3. The same trend was also found in the DOPV analysis. The surfactant concentration influenced the bubble interface properties relating to the oxygen transfer process. Higher surfactant concentration would exert larger mass transfer resistance on the interface.
The gas type played a major role in the parameter ST, that is to say oxygen bubbles, regardless of sizes, had a greater durability performance than air bubbles (at least four times longer). Groups 3, 5 and 6 indicated that surfactant extended the stagnation time probably because the surfactant slowed down the bubble dissolving process.
From the results above, it was clear that the surfactant has an impact on the gas-water interface properties which would not only influence the bubble formation process, as mentioned in
Section 3.1, but also the physical behavior. In a word, higher surfactant concentration would make the bubble size smaller, the oxygen transfer efficiency lower and the stagnation time of MNBs in water longer. Therefore, whether the surfactant needs to be added into water for the MNBs formation has to be carefully taken into consideration.