The Study of External Dose Rate and Retained Body Activity of Patients Receiving 131I Therapy for Differentiated Thyroid Carcinoma
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients’ Materials
2.2. Measurement of the EDR
2.3. Estimation of the RBA
3. Results and Discussion
3.1. EDR

3.2. The Point Resource

3.3. RBA

| Group | Activity Administered to Patients (MBq) | ||
|---|---|---|---|
| 1850 | 3700 | 5550 | |
| group (A) | 22.3 | 33.4 | 39.9 |
| group (FU) | 21.5 | 32.1 | 38.8 |
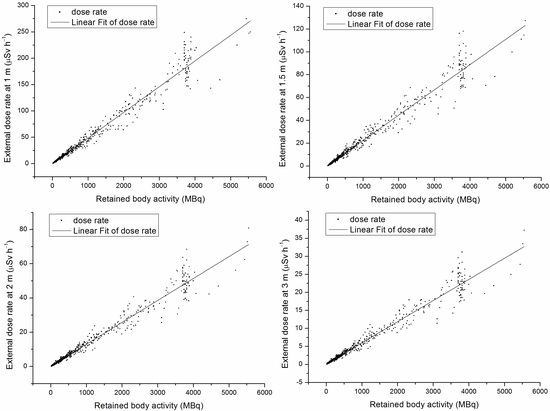
3.4. The Relationship between EDR and RBA

3.5. Normalized Cumulative Dose
| Group | Distance | Period of Time Post 131I Administration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–6 h | 6–12 h | 12–18 h | 18–24 h | 24–30 h | 30–36 h | 36–42 h | 42–48 h | 48–54 h | 54–60 h | 60–66 h | 66–72 h | ||
| group (A) | 1 m | 0.25763 | 0.15540 | 0.10409 | 0.07081 | 0.04827 | 0.03292 | 0.02245 | 0.01531 | 0.01044 | 0.00712 | 0.00485 | 0.00331 |
| 1.5 m | 0.12358 | 0.07414 | 0.05059 | 0.03452 | 0.02356 | 0.01607 | 0.01097 | 0.00748 | 0.00511 | 0.00348 | 0.00238 | 0.00162 | |
| 2 m | 0.07074 | 0.04256 | 0.02899 | 0.01974 | 0.01345 | 0.00916 | 0.00624 | 0.00425 | 0.00289 | 0.00197 | 0.00134 | 0.00091 | |
| 3 m | 0.03272 | 0.01942 | 0.01303 | 0.00874 | 0.00587 | 0.00394 | 0.00264 | 0.00177 | 0.00119 | 0.00080 | 0.00054 | 0.00036 | |
| group (FU) | 1 m | 0.23573 | 0.14137 | 0.09405 | 0.06408 | 0.04387 | 0.03007 | 0.02062 | 0.01413 | 0.00969 | 0.00665 | 0.00456 | 0.00312 |
| 1.5 m | 0.10692 | 0.06805 | 0.04596 | 0.03108 | 0.02102 | 0.01421 | 0.00961 | 0.00650 | 0.00440 | 0.00297 | 0.00201 | 0.00136 | |
| 2 m | 0.06280 | 0.03941 | 0.02622 | 0.01789 | 0.01232 | 0.00852 | 0.00591 | 0.00409 | 0.00284 | 0.00197 | 0.00137 | 0.00095 | |
| 3 m | 0.02903 | 0.01854 | 0.01220 | 0.00822 | 0.00565 | 0.00394 | 0.00278 | 0.00197 | 0.00141 | 0.00101 | 0.00073 | 0.00052 | |
3.6. Home Care Precautions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oliver, S.; Peter, H.; ∅yvind, S.B. Targeted radio-nuclide therapy of skeletal metastases. Cancer Treat. Rev. 2013, 39, 18–26. [Google Scholar]
- Turner, J.H. Outpatient therapeutic nuclear oncology. Ann. Nucl. Med. 2012, 26, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Mier, W.; Kratochwil, C.; Hassel, J.C.; Giesel, F.L.; Beijer, B.; Babich, J.W.; Friebe, M.; Eisenhut, M.; Enk, A.; Haberkorn, U. Radiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. J. Nucl. Med. 2014, 55, 9–14. [Google Scholar] [CrossRef] [PubMed]
- International Commissioin on Radiological Protection. ICRP Publication 94, Release of Patients after Therapy with Unsealed Radionuclides; Pergamon Press: Oxford, UK, 2004. [Google Scholar]
- State Standard of the People’s Republic of China. GB 18871–2002: Basic Standard for Protection against Ionizing Radiation and for the Safety of Radiation Sources; Chinese Standard Press: Beijing, China, 2003; pp. 35–37. [Google Scholar]
- International Atomic Energy Agency. Nuclear Medicine Resources Manual; International Atomic Energy Agency: Vienna, Austria, 2006. [Google Scholar]
- European Commission. Radiation Protection 97, Radiation Protectioin Following Iodine-131 Therapy (Exposures due to Out-Patients or Discharged In-Patient); European Commission: Luxembourg, Luxembourg, 1998. [Google Scholar]
- Faraj, T.; Isa, N.A.; Zahra, A.; Hamid, J.; Majid, A. Assessment of radioiodine clearance in patients with differentiated thyroid cancer. Radiat. Prot. Dosim. 2012, 152, 323–327. [Google Scholar] [CrossRef]
- Barrington, S.F.; Kettle, A.G.; O’Doherty, M.J.; Wells, C.P.; Somer, E.J.R.; Coakley, A.J. Radiation dose rates from patients receiving iodine-131 therapy for carcinoma of the thyroid. Eur. J. Nucl. Med. 1996, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Willegaignon, J.; Stabin, M.G.; Guimaraes, M.I.C.; Malvestiti, L.F.; Sapienza, M.T.; Maroni, M.; Sordi, G.A.A. Evaluation of the potential absorbed doses from patients based on whole-body 131I clearance in thyroid cancer therapy. Health Phys. 2006, 91, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Fioroni, F.; Sghedoni, R.; Grassi, E.; Piccagli, V.; Sollini, M.; Filice, A.; Versari, A.; Iori, M. Radiation protection procedures in 131I treatments for thyroid cancer in patients requiring hemodialy. Nucl. Med. Commun. 2014, 35, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Parlak, Y.; Cavdar, I.; Yeyin, N.; Tanyildizi, H.; Gumuser, G.; Sayit, E.; Erees, S.; Sayman, H. The evaluation of urine activity and external dose rate from patients receiving radioiodine therapy for thyroid cancer. Radiat. Prot. Dosim. 2013, 156, 25–29. [Google Scholar] [CrossRef]
- American Thyroid Association Taskforce on Radioiodine Safety; Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.T.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; et al. Radiation safety in the measurement of patients with thyroid diseases by radioiodine 131I: Practice recommendations of the American Thtyroid Association. Thyroid 2011, 21, 335–346. [Google Scholar]
- Al-Haj, A.N.; Lagarde, C.S.; Lobriguito, A.M. Patient parameters and other radiation safety issues in 131I therapy for thyroid cancer treatment. Health Phys. 2007, 93, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, D.; Atkins, F.; Bandaru, V.V.; Chennupati, S.P.; Moreau, S.; Burman, K.; Wartofsky, L. Salivary gland protection with sialagogues: A case study. Thyroid 2009, 19, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Marcus, C.S.; Sparks, R.B. Calculating the absorbed dose from radioactive patients: The line-source versus point-source model. J. Nucl. Med. 2002, 43, 1241–1244. [Google Scholar] [PubMed]
- Cui, S.; Jiao, L.; Tan, J.; Zhang, G.; Zhang, H.; Long, W.; Fan, S.; Zhang, W. Estimating radiation absorbed dose of individuals nearby 131I-treated hyperthyroid patients. Health Phys. 2014, 106, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Marcus, C.S.; Stabin, M.G. Licensee over-reliance on conservatisms in NRC guidance regarding the release of patients treated with 131I. Health Phys. 2007, 93, 667–677. [Google Scholar] [CrossRef]
- Muhammad, W.; Faaruq, S.; Matiullah; Hussain, A.; Khan, A.A. Release criteria from hospitals of 131I thyrotoxicosis therapy patients in developing countries—Case study. Radiat. Prot. Dosim. 2006, 121, 136–139. [Google Scholar] [CrossRef]
- Andres, C.; Barquero, R.; Tortosa, R.; Nunez, C.; del Castillo, A.; Vega-Carrillo, H.R.; Alonso, D. 131I activity in urine to the sewer system due to thyroidal treatments. Health Phys. 2011, 101, S110–S115. [Google Scholar] [CrossRef]
- Thomas, S.R.; Maxon, H.R.; Fritz, K.M.; Kereiakes, J.G.; Connell, W.D. A comparison of methods for assessing patient body burden following 131I therapy for thyroid cancer. Radiology 1980, 137, 839–842. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jiao, L.; Cui, S.; Wang, L.; Tan, J.; Zhang, G.; He, Y.; Ruan, S.; Fan, S.; Zhang, W. The Study of External Dose Rate and Retained Body Activity of Patients Receiving 131I Therapy for Differentiated Thyroid Carcinoma. Int. J. Environ. Res. Public Health 2014, 11, 10991-11003. https://doi.org/10.3390/ijerph111010991
Zhang H, Jiao L, Cui S, Wang L, Tan J, Zhang G, He Y, Ruan S, Fan S, Zhang W. The Study of External Dose Rate and Retained Body Activity of Patients Receiving 131I Therapy for Differentiated Thyroid Carcinoma. International Journal of Environmental Research and Public Health. 2014; 11(10):10991-11003. https://doi.org/10.3390/ijerph111010991
Chicago/Turabian StyleZhang, Haiying, Ling Jiao, Songye Cui, Liang Wang, Jian Tan, Guizhi Zhang, Yajing He, Shuzhou Ruan, Saijun Fan, and Wenyi Zhang. 2014. "The Study of External Dose Rate and Retained Body Activity of Patients Receiving 131I Therapy for Differentiated Thyroid Carcinoma" International Journal of Environmental Research and Public Health 11, no. 10: 10991-11003. https://doi.org/10.3390/ijerph111010991