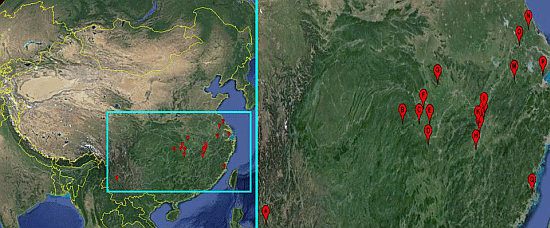
Sensitivity of Oncomelania hupensis to Niclosamide: A Nation-Wide Survey in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Snails
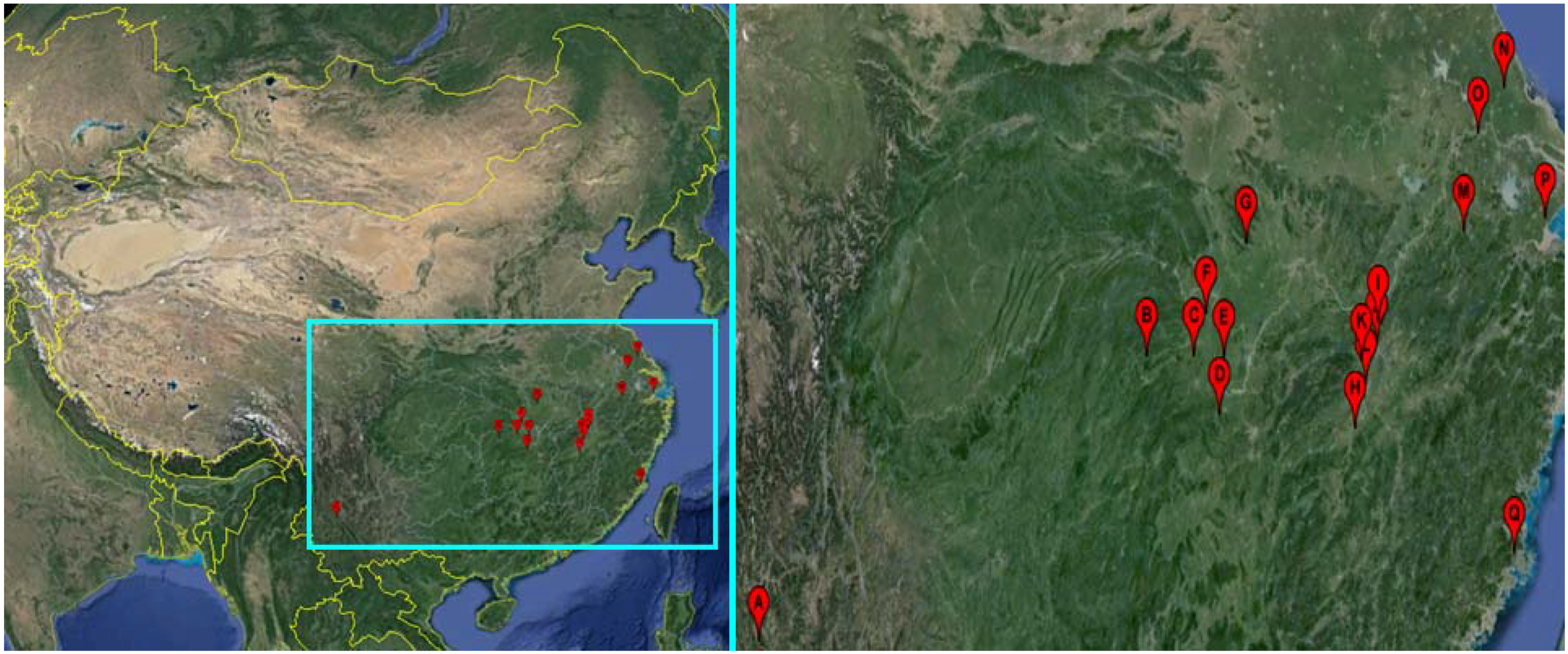
| Snail Sampling Site | Environmental Type | Code | East Longitude | North Latitude |
|---|---|---|---|---|
| Weishan County, Yunnan Province | Hill | A | 100.33° | 25.23° |
| Shimen County, Hunan Province | Hill | B | 110.47° | 29.51° |
| Linli County, Hunan Province | Hill | C | 111.64° | 29.44° |
| Yuanjiang County, Hunan Province | Lake and marshland | D | 112.20° | 28.52° |
| Nanxian County, Hunan Province | Lake and marshland | E | 112.39° | 29.37° |
| Gong’an County, Hubei Province | Lake and marshland | F | 112.00° | 30.05° |
| Jingshan County, Hubei Province | Hill | G | 113.11° | 31.03° |
| Wucheng County, Jiangxi Province | Lake and marshland | H | 115.54° | 28.05° |
| Pengze County, Jiangxi Province | Lake and marshland | I | 116.32° | 29.58° |
| Duchang County, Jiangxi Province | Lake and marshland | J | 116.24° | 29.25° |
| Yongxiu County, Jiangxi Province | Lake and marshland | K | 115.82° | 29.04° |
| Nanchang County, Jiangxi Province | Lake and marshland | L | 115.89° | 28.68° |
| Xuancheng County, Anhui Province | Hill | M | 118.77° | 30.74° |
| Dongtai County, Jiangsu Province | Plain with waterway networks | N | 120.31° | 32.84° |
| Zhenjiang County, Jiangsu Province | Plain with waterway networks | O | 119.44° | 32.20° |
| Pinghu County, Zhejiang Province | Plain with waterway networks | P | 121.02° | 30.70° |
| Yinxi County, Fujian Province | Hill | Q | 119.35° | 25.72° |
2.2. Niclosamide Formulation
2.3. Molluscicidal Test
2.4. Statistical Analysis
3. Results
| Snail Population | Mortality of Snails in Different Concentrations of WPN | LC50 (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 mg/L | 0.5 mg/L | 0.25 mg/L | 0.125 mg/L | 0.063 mg/L | 0.032 mg/L | 0.016 mg/L | 0.008 mg/L | ||
| Weishan | 100 | 100 | 90 | 20 | 15 | 5 | 0 | 0 | 0.1436 |
| Shimen | 100 | 100 | 80 | 80 | 50 | 10 | 0 | 0 | 0.0770 |
| Linli | 100 | 95 | 75 | 15 | 10 | 5 | 5 | 0 | 0.1708 |
| Ruanjiang | 100 | 95 | 95 | 70 | 25 | 0 | 0 | 0 | 0.0981 |
| Nanxian | 100 | 80 | 65 | 20 | 5 | 0 | 0 | 0 | 0.2177 |
| Gong’an | 100 | 100 | 63.33 | 3.33 | 0 | 0 | 0 | 0 | 0.2222 |
| Jingshan | 100 | 100 | 93.33 | 6.67 | 6.67 | 0 | 0 | 0 | 0.1684 |
| Wucheng | 100 | 100 | 100 | 83.33 | 26.67 | 6.67 | 3.33 | 0 | 0.0770 |
| Pengze | 100 | 96.67 | 76.67 | 20 | 0 | 0 | 0 | 0 | 0.1856 |
| Duchang | 100 | 100 | 100 | 46.67 | 20 | 0 | 0 | 0 | 0.1115 |
| Yongxiu | 100 | 100 | 100 | 80 | 30 | 15 | 0 | 0 | 0.0743 |
| Nanchang | 100 | 100 | 100 | 85 | 15 | 5 | 0 | 0 | 0.0854 |
| Xuancheng | 100 | 96.67 | 66.67 | 0 | 0 | 0 | 0 | 0 | 0.2285 |
| Dongtai | 100 | 100 | 90 | 5 | 0 | 0 | 0 | 0 | 0.1830 |
| Zhenjiang | 100 | 100 | 77 | 27 | 20 | 0 | 0 | 0 | 0.1497 |
| Pinghu | 100 | 100 | 86.67 | 23.33 | 3.33 | 3.33 | 0 | 0 | 0.1575 |
| Yinxi | 100 | 100 | 100 | 10 | 0 | 0 | 0 | 0 | 0.1649 |
| Snail Population | Mortality of Snails in Different Concentrations of WPN | LC50 (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 mg/L | 0.5 mg/L | 0.25 mg/L | 0.125 mg/L | 0.063 mg/L | 0.032 mg/L | 0.016 mg/L | 0.008 mg/L | ||
| Weishan | 100 | 100 | 100 | 70 | 10 | 5 | 0 | 0 | 0.1015 |
| Shimen a | – | – | – | – | – | – | – | – | – |
| Linli | 100 | 100 | 100 | 25 | 20 | 5 | 5 | 0 | 0.1208 |
| Ruanjiang | 100 | 100 | 100 | 75 | 35 | 0 | 0 | 0 | 0.0825 |
| Nanxian | 100 | 100 | 95 | 75 | 15 | 0 | 0 | 0 | 0.0981 |
| Gong’an | 100 | 100 | 100 | 60 | 10 | 0 | 0 | 0 | 0.1088 |
| Jingshan | 100 | 100 | 100 | 40 | 3.33 | 3.33 | 0 | 0 | 0.1309 |
| Wucheng | 100 | 100 | 100 | 90 | 13.33 | 3.33 | 0 | 0 | 0.0864 |
| Pengze | 100 | 100 | 100 | 63.33 | 10 | 0 | 0 | 0 | 0.1063 |
| Duchang | 100 | 100 | 100 | 80 | 33.33 | 0 | 0 | 0 | 0.0806 |
| Yongxiu | 100 | 100 | 100 | 100 | 55 | 30 | 0 | 0 | 0.0490 |
| Nanchang | 100 | 100 | 100 | 100 | 30 | 0 | 0 | 0 | 0.0718 |
| Xuancheng | 100 | 100 | 96.67 | 16.67 | 0 | 3.33 | 0 | 0 | 0.1575 |
| Dongtai | 100 | 100 | 100 | 25 | 0 | 0 | 0 | 0 | 0.1487 |
| Zhenjiang | 100 | 100 | 97 | 57 | 13 | 10 | 0 | 0 | 0.1037 |
| Pinghu | 100 | 100 | 96.67 | 23.33 | 0 | 0 | 0 | 0 | 0.1539 |
| Yinxi | 100 | 100 | 100 | 10 | 5 | 0 | 0 | 0 | 0.1593 |
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interests
References
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Wang, L.D.; Utzinger, J.; Zhou, X.N. Schistosomiasis control: Experiences and lessons from China. Lancet 2008, 372, 1793–1795. [Google Scholar] [CrossRef]
- Manus, D.P.; Li, Y.; Gray, D.J.; Ross, A.G. Conquering “snail fever”: Schistosomiasis and its control in China. Expert Rev. Anti Infect. Ther. 2009, 7, 473–485. [Google Scholar] [CrossRef]
- Utzinger, J.; Zhou, X.N.; Chen, M.G.; Bergquist, R. Conquering Schistosomiasis in China: The long march. Acta Trop. 2005, 96, 69–96. [Google Scholar] [CrossRef]
- Zhou, X.N.; Guo, J.G.; Wu, X.H.; Jiang, Q.W.; Zheng, J.; Dang, H.; Wang, X.H.; Xu, J.; Zhu, H.Q.; Wu, G.L.; et al. Epidemiology of Schistosomiasis in the People’s Republic of China, 2004. Emerg. Infect. Dis. 2007, 13, 1470–1476. [Google Scholar] [CrossRef]
- Li, S.Z.; Luz, A.; Wang, X.H.; Xu, L.L.; Wang, Q.; Qian, Y.J.; Wu, X.H.; Guo, J.G.; Xia, G.; Wang, L.Y.; Zhou, X.N. Schistosomiasis in China: Acute infections during 2005–2008. Chin. Med. J. 2009, 122, 1009–1014. [Google Scholar]
- Zheng, H.; Zhang, L.J.; Zhu, R.; Xu, J.; Li, S.Z.; Guo, J.G.; Xiao, N.; Zhou, X.N. Schistosomiasis situation in People’s Republic of China in 2011. Chin. J. Schisto. Control 2012, 24, 621–626. (in Chinese). [Google Scholar]
- Zhou, X.N.; Wang, L.Y.; Chen, M.G.; Wu, X.H.; Jiang, Q.W.; Chen, X.Y.; Zheng, J.; Utzinger, J. The public health significance and control of Schistosomiasis in China—Then and now. Acta Trop. 2005, 96, 97–105. [Google Scholar] [CrossRef]
- Zhou, X.N.; Bergquist, R.; Leonardo, L.; Yang, G.J.; Yang, K.; Sudomo, M.; Olveda, R. Schistosomiasis japonica control and research needs. Adv. Parasitol. 2010, 72, 145–178. [Google Scholar] [CrossRef]
- Wang, L.D.; Guo, J.G.; Wu, X.H.; Chen, H.G.; Wang, T.P.; Zhu, S.P.; Zhang, Z.H.; Steinmann, P.; Yang, G.J.; Wang, S.P.; Wu, Z.D.; Wang, L.Y.; Hao, Y.; Bergquist, R.; Utzinger, J.; Zhou, X.N. China’s new strategy to block Schistosoma. japonicu transmission: Experiences and impact beyond schistosomiasis. Trop. Med. Int. Health 2009, 14, 1475–1483. [Google Scholar] [CrossRef]
- Zhou, X.N. Science of Oncomelania Snail; (in Chinese). Science Press: Beijing, China, 2005; pp. 97–108, 317–318. [Google Scholar]
- Yuan, Y.; Xu, X.J.; Dong, H.F.; Jiang, M.S.; Zhu, H.G. Transmission control of Schistosomiasis japonica: Implementation and evaluation of different snail control interventions. Acta Trop. 2005, 96, 191–197. [Google Scholar] [CrossRef]
- Lin, D.D.; Hu, G.H.; Zhang, S.J. Optimal combined approaches of field intervention for Schistosomiasis control in China. Acta Trop. 2005, 96, 242–247. [Google Scholar] [CrossRef]
- WHO. The Role of Mollusciciding in Schistosomiasis Control; Division of Control of Tropical Diseases: Geneva, Switzerland, 1992; pp. 1–10. [Google Scholar]
- Xianyi, C.; Liying, W.; Jiming, C.; Xiaonong, Z.; Jiang, Z.; Jiagang, G.; Xiaohua, W.; Engels, D.; Minggang, C. Schistosomiasis control in China: The impact of a 10-year World Bank Loan Project (1992–2001). Bull WHO 2005, 83, 43–48. [Google Scholar]
- Cao, Z.G.; Wang, T.P.; Zhang, S.Q.; Tian, X.G.; Zhu, L.; Zhang, L.S.; Yao, G.X.; Jin, W.; Yang, W.P. Experimental study on the resistance of Oncomelania snails to niclosamide. Chin. J. Pathogen Biol. 2012, 7, 352–353, 376. (in Chinese). [Google Scholar]
- Mao, C.P. Biology of Schistosome and Control of Schistosomiasis; (in Chinese). People’s Medical Publishing House: Beijing, China, 1990. [Google Scholar]
- Webbe, G. Laboratory and field trials of a new molluscicide, Bayer 73, in Tanganyika. Bull WHO 1961, 25, 525–531. [Google Scholar]
- Gönnert, R. Results of laboratory and field trials with the molluscicide Bayer 73. Bull WHO 1961, 25, 483–501. [Google Scholar]
- Yang, G.J.; Sun, L.P.; Hong, Q.B.; Zhu, H.R.; Yang, K.; Gao, Q.; Zhou, X.N. Optimizing molluscicide treatment strategies in different control stages of Schistosomiasis in the People’s Republic of China. Parasit. Vector. 2012, 5. [Google Scholar] [CrossRef]
- Yang, G.J.; Li, W.; Sun, L.P.; Wu, F.; Yang, K.; Huang, Y.X.; Zhou, X.N. Molluscicidal efficacies of different formulations of niclosamide: Result of meta-analysis of Chinese literature. Parasit. Vectors 2010, 3. [Google Scholar] [CrossRef]
- Zhou, P.S.; Ma, L.; Huang, J.L. Research progress on molluscicides and their application. Chin. J. Vector Biol. Control 2002, 13, 231–233. (in Chinese). [Google Scholar]
- Wu, X.Y.; Yang, L.Q.; Zhang, L.H.; Ge, Q.J. Progress of research on molluscicides. Chin. J. Schistosomiasis Control 2006, 18, 474–476. (in Chinese). [Google Scholar]
- Zhu, D.; Yao, P.; Bao, Z. Mollusciciding action and toxicity of bromoacetamide. Chin. J. Parasitol. Parasit. Dis. 1999, 17, 244–246. (in Chinese). [Google Scholar]
- Zhu, D.; Zhou, X.N.; Zhang, S.Q.; Zhang, G.; Liu, H.X.; Lu, D.B.; Cai, G.Y.; Ni, Q.Z.; Cao, Z.G.; Wu, W.D. Study on the molluscicidal effect of META-Li against Oncomelania hupensis. Chin. J. Parasitol. Parasit. Dis. 2006, 17, 244–246. (in Chinese). [Google Scholar]
- Wei, F.H.; Xu, X.J.; Liu, J.B.; Dai, Y.H.; Dussart, G.; Trigwell, J. Toxicology of a potential molluscicide derived from the plant Solanum xanthocarpum: A preliminary study. Ann. Trop. Med. Parasitol. 2002, 96, 325–331. [Google Scholar] [CrossRef]
- Wang, H.; Cai, W.M.; Wang, W.X.; Yang, J.M. Molluscicidal activity of Nerium indicum Mill, Pterocarya stenoptera DC, and Rumex japonicum houtt on Oncomelania hupensis. Biomed. Environ. Sci. 2006, 19, 245–248. [Google Scholar]
- Yang, X.M.; Chen, S.X.; Xia, L.; Chen, J. Molluscicidal activity against Oncomelania hupensis of Ginkgo biloba. Fitoterapia 2008, 89, 250–254. [Google Scholar] [CrossRef]
- Zou, F.C.; Duan, G.; Xie, Y.J.; Zhou, Y.; Dong, G.D.; Lin, R.Q.; Zhu, X.Q. Molluscicidal activity of the plant Eupatorium adenophorum against Oncomelania hupensis, the intermediate host snail of Schistosoma japonicum. Ann. Trop. Med. Parasitol. 2009, 103, 549–553. [Google Scholar] [CrossRef]
- Peng, F.; Liu, M.; Huang, Q.; Liu, N.; Yang, H.; Sun, H.; Hu, Q.; Feng, F.; Jiang, C. Molluscicidal effect of Eomecon chionantha alkaloids against Oncomelania hupensis snails. Southeast Asian J. Trop. Med. Public Health 2011, 42, 289–296. [Google Scholar]
- Dai, J.R.; Zhang, Y.P.; Jiang, Y.J.; Xi, W.P.; Yang, G.J.; Liang, Y.S. Studies on standardization of methods for screening molluscicides in laboratory I Volume of molluscicidal solution to influence the efficacy. Chin. J. Schisto. Control 2002, 14, 122–124. (in Chinese). [Google Scholar]
- Dai, J.R.; Xi, W.P.; Zhang, Y.P.; Liang, Y.S. Studies on standardization of methods for screening molluscicides in laboratory II Quantity of snails used to influence the efficacy. Chin. J. Schisto. Control. 2002, 14, 263–265. (in Chinese). [Google Scholar]
- Dai, J.R.; Liang, Y.S.; Zhang, Y.P.; Xu, M.; Li, H.J.; Zhu, Y.C. Studies on standardization of methods for screening molluscicides in laboratory III Breeding time of snails to influence the efficacy. Chin. J. Schisto. Control. 2003, 14, 346–348. (in Chinese). [Google Scholar]
- Li, Y.Z.; Xing, Y.T.; Li, H.J.; Qu, G.L.; Wang, W.; Wei, J.Y.; Liang, Y.S.; Dai, J.R. Studies on standardization of methods for screening molluscicides in laboratory IV breeding time of snails to influence the efficacy. Chin. J. Schisto. Control. 2012, 24, 35–39. (in Chinese). [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dai, J.; Li, Y.; Wang, W.; Xing, Y.; Qu, G.; Liang, Y. Sensitivity of Oncomelania hupensis to Niclosamide: A Nation-Wide Survey in China. Int. J. Environ. Res. Public Health 2014, 11, 3086-3095. https://doi.org/10.3390/ijerph110303086
Dai J, Li Y, Wang W, Xing Y, Qu G, Liang Y. Sensitivity of Oncomelania hupensis to Niclosamide: A Nation-Wide Survey in China. International Journal of Environmental Research and Public Health. 2014; 11(3):3086-3095. https://doi.org/10.3390/ijerph110303086
Chicago/Turabian StyleDai, Jianrong, Youzi Li, Wei Wang, Yuntian Xing, Guoli Qu, and Yousheng Liang. 2014. "Sensitivity of Oncomelania hupensis to Niclosamide: A Nation-Wide Survey in China" International Journal of Environmental Research and Public Health 11, no. 3: 3086-3095. https://doi.org/10.3390/ijerph110303086
APA StyleDai, J., Li, Y., Wang, W., Xing, Y., Qu, G., & Liang, Y. (2014). Sensitivity of Oncomelania hupensis to Niclosamide: A Nation-Wide Survey in China. International Journal of Environmental Research and Public Health, 11(3), 3086-3095. https://doi.org/10.3390/ijerph110303086