Influence of Heavy Metals and PCBs Pollution on the Enzyme Activity and Microbial Community of Paddy Soils around an E-Waste Recycling Workshop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling

2.2. Chemical Analysis
2.3. Soil Enzyme Activity Analysis
2.4. Microbial Counts
2.5. PCR-DGGE Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Soil Properties, Heavy Metals and PCBs Analysis
| Sampling No. | Distance (m) a | Zn | Cu | Pb | Cd | Cr | Ni |
|---|---|---|---|---|---|---|---|
| P1 | 5 | 95.44 ± 9.78 a | 37.17 ± 3.07 a | 87.22 ±7 .69 a | 1.83 ± 0.28 ab | 60.17 ± 5.33 a | 30.77 ± 2.39 a |
| P2 | 10 | 126.53 ± 10.17 b | 81.14 ± 0.64 b | 92.16 ± 7.82 ab | 2.08 ± 0.33 abc | 68.17 ± 4.12 b | 32.70 ± 1.17 ab |
| P3 | 15 | 134.50 ± 8.71 bc | 78.26 ± 0.67 c | 107.10 ± 9.40 c | 2.56 ± 0.59 c | 74.18 ± 1.11 b | 35.58 ± 0.76 c |
| P4 | 20 | 129.00 ± 9.26 bc | 71.36 ± 0.55 d | 99.54 ± 6.15 abc | 2.57 ± 0.34 a | 69.04 ± 3.60 b | 35.37 ± 1.84 bc |
| P5 | 25 | 131.34 ± 4.12 bc | 68.59 ± 0.57 d | 92.22 ± 4.30 ab | 1.71 ± 0.24 b | 71.11 ± 3.18 b | 34.89 ± 0.66 bc |
| P6 | 30 | 142.00 ± 12.73 c | 78.94 ± 0.14 bc | 103.98 ± 3.61 bc | 2.23 ± 0.19 abc | 73.53 ± 1.84 b | 38.93 ± 1.60 d |

3.2. Enzyme Activities
| Sampling No. | Distance (m) | Catalase (mL·g−1·dw) | Urease (mg∙kg−1·24∙h−1∙dw) | Phosphatase (mg∙kg−1·h−1·dw) |
|---|---|---|---|---|
| P1 | 5 | 4.27 ± 0.17 a | 182.56 ± 12.82 a | 66.65 ± 11.05 a |
| P2 | 10 | 5.97 ± 0.18 b | 290.93 ± 20.17 b | 100.27 ± 7.46 b |
| P3 | 15 | 6.28 ± 0.26 bc | 385.35 ± 26.24 c | 186.98 ± 33.56 cd |
| P4 | 20 | 5.79 ± 0.13 b | 290.74 ± 1.61 b | 222.90 ± 19.87 ce |
| P5 | 25 | 6.72 ± 0.07 c | 309.18 ± 7.26 b | 231.49 ± 16.88 e |
| P6 | 30 | 6.75 ± 0.75 c | 339.96 ± 14.79 d | 189.32 ± 17.20 d |
3.3. Microbial Counts
| Sampling No. | Distance (m) | Bacteria (106/g·dw) | Actinomycetes (106/g·dw) |
|---|---|---|---|
| P1 | 5 | 0.40 ± 0.06 ab | 0.19 ± 0.01 ab |
| P2 | 10 | 0.38 ± 0.07 ab | 0.32 ± 0.03 a |
| P3 | 15 | 0.54 ± 0.19 ac | 0.23 ± 0.02 ab |
| P4 | 20 | 0.59 ± 0.16 ac | 0.12 ± 0.01 b |
| P5 | 25 | 0.19 ± 0.16 b | 0.22 ± 0.12 ab |
| P6 | 30 | 0.69 ± 0.09 c | 0.21 ± 0.01 ab |
3.4. Microbial Community Structure Analysis


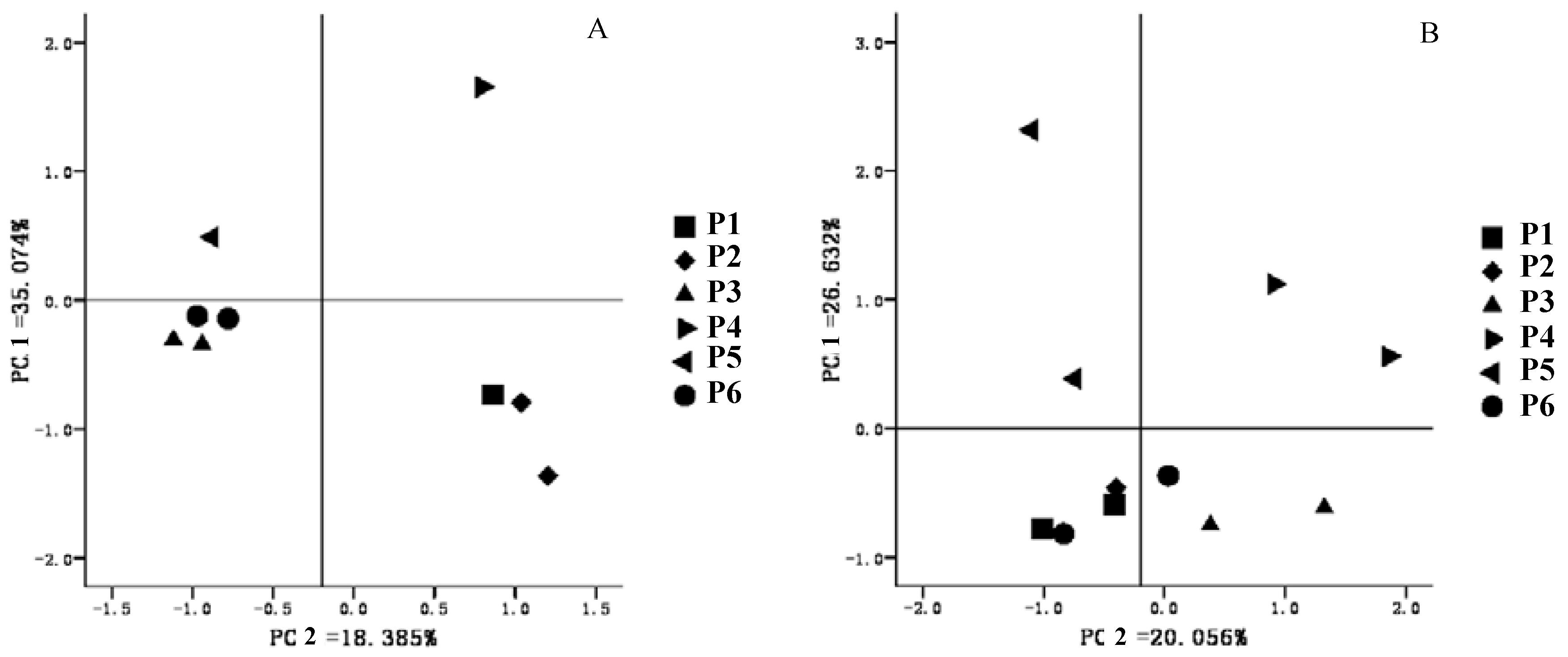
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, M.H.; Wu, S.C.; Deng, W.J.; Yu, X.Z.; Luo, Q.; Leung, A.O.W.; Wong, C.S.C.; Luksemburg, W.J.; Wong, A.S. Export of toxic chemicals—A review of the case of uncontrolled electronic-waste recycling. Environ. Pollut. 2007, 149, 131–140. [Google Scholar] [CrossRef]
- Chen, D.; Bi, X.; Zhao, J.; Chen, L.; Tan, J.; Mai, B.; Sheng, G.; Fu, J.; Wong, M. Pollution characterization and diurnal variation of PBDEs in the atmosphere of an e-waste dismantling region. Environ. Pollut. 2009, 157, 1051–1057. [Google Scholar] [CrossRef]
- Bi, X.; Simoneit, B.R.T.; Wang, Z.Z.; Wang, X.; Sheng, G.; Fu, J. The major components of particles emitted during recycling of waste printed circuit boards in a typical e-waste workshop of South China. Atmos. Environ. 2010, 44, 4440–4445. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, C.; Li, J.; Yin, H.; Li, X.; Zhang, G. Characterization of PBDEs in soils and vegetations near an e-waste recycling site in South China. Environ. Pollut. 2011, 159, 2443–2448. [Google Scholar] [CrossRef]
- Luo, C.; Liu, C.; Wang, Y.; Liu, X.; Li, F.; Zhang, G.; Li, X. Heavy metal contamination in soils and vegetables near an e-waste processing site, South China. J. Hazard. Mater. 2011, 186, 481–490. [Google Scholar] [CrossRef]
- Shen, C.; Chen, Y.; Huang, S.; Wang, Z.; Yu, C.; Qiao, M.; Xu, Y.; Setty, K.; Zhang, J.; Zhu, Y. Dioxin-like compounds in agricultural soils near e-waste recycling sites from Taizhou area, China: Chemical and bioanalytical characterization. Environ. Int. 2009, 35, 50–55. [Google Scholar] [CrossRef]
- Tang, X.J.; Shen, C.; Chen, L.; Xiao, X.; Khan, M.I.; Dou, C.; Chen, Y.X. Inorganic and organic pollution in agricultural soil from an emerging e-waste recycling town in Taizhou area, China. J. Soil Sediment. 2010, 10, 895–906. [Google Scholar] [CrossRef]
- Filip, Z. International approach to assessing soil quality by ecologically-related biological parameters. Agri. Eco. Environ. 2002, 88, 169–174. [Google Scholar] [CrossRef]
- Wang, Y.P.; Shi, J.Y.; Wang, H.; Lin, Q.; Chen, X.C.; Chen, Y.X. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotox. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef]
- Microorganisms as Indicators of Soil Health. Available online: http://www.neri.dk/1_viden/2_Publikationer/3_fagrapporter/rapporter/FR388.pdf (accessed on 27 December 2013).
- Killham, K.; Staddon, W.J. Bioindicators and Sensors of Soil Health and the Application of Geostatistics. In Enzymes in the Environment; Burns, R.G., Dick, R.P., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 391–405. [Google Scholar]
- Tu, C.; Teng, Y.; Luo, Y.; Sun, X.; Deng, S.; Li, Z.; Liu, W.; Xu, Z. PCB removal, soil enzyme activities, and microbial community structures during the phytoremediation by alfalfa in field soils. J. Soil Sediment. 2011, 11, 649–656. [Google Scholar] [CrossRef]
- Thavamani, P.; Malik, S.; Beer, M.; Megharaj, M.; Naidu, R. Microbial activity and diversity in long-term mixed contaminated soils with respect to polyaromatic hydrocarbons and heavy metals. J. Environ. Manage. 2012, 99, 10–17. [Google Scholar] [CrossRef]
- Renella, G.; Mench, M.; Landi, L.; Nannipieri, P. Microbial activity and hydrolase synthesis in long-term Cd-contaminated soils. Soil Biol. Biochem. 2005, 37, 133–139. [Google Scholar] [CrossRef]
- Suhadolc, M.; Schroll, R.; Gattinger, A.; Schloter, M.; Munch, J.C.; Lestan, D. Effects of modified Pb-, Zn-, and Cd-availability on the microbial communities and on the degradation of isoproturon in a heavy metal contaminated soil. Soil Biol. Biochem. 2004, 36, 1943–1954. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J.; Lombi, E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant. Soil 2001, 232, 207–214. [Google Scholar] [CrossRef]
- Lasat, M.M. Phytoextraction of toxic metals: A review of biological mechanisms. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Shin, S.G.; O’Flaherty, V.; Hwang, S. Quantitative and qualitative transitions of methanogen community structure during the batch anaerobic digestion of cheese-processing wastewater. Appl. Microbio. Biotech. 2010, 87, 1963–1973. [Google Scholar] [CrossRef]
- Baker, G.C.; Gaffar, S.; Cowan, D.A.; Suharto, A.R. Bacterial community analysis of Indonesian hot springs. FEMS Microbiol. Lett. 2001, 200, 103–109. [Google Scholar] [CrossRef]
- Muyzer, G.; Smalla, K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie van Leeuwenhoek Int J. Gen. Molec. Microbiol. 1998, 73, 127–141. [Google Scholar] [CrossRef]
- Kim, S.; Baek, K.; Lee, I. Phytoremediation and microbial community structure of soil from a metal-contaminated military shooting range: Comparisons of field and pot experiments. J. Environ. Sci. Heal. A 2010, 45, 389–394. [Google Scholar] [CrossRef]
- Lopes, A.R.; Faria, C.; Prieto-Fernandez, A.; Trasar-Cepeda, C.; Manaia, C.M.; Nunes, O.C. Comparative study of the microbial diversity of bulk paddy soil of two rice fields subjected to organic and conventional farming. Soil Biol. Biochem. 2011, 43, 115–125. [Google Scholar] [CrossRef]
- Tang, X.; Qiao, J.; Chen, C.; Chen, L.; Yu, C.; Shen, C.; Chen, Y. Bacterial communities of polychlorinated biphenyls polluted soil around an e-waste recycling workshop. Soil Sedimet Contam. 2013, 22, 562–573. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. (in Chinese) [Google Scholar]
- Lu, R.K. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. (in Chinese) [Google Scholar]
- Hseu, Z.Y.; Chen, Z.S.; Tsai, C.C.; Tsui, C.C.; Cheng, S.F.; Liu, C.L.; Lin, H.T. Digestion methods for total heavy metals in sediments and soils. Water Air Soil Pollut. 2002, 141, 189–205. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzyme and Research Methods; China Agriculture Press: Beijing, China, 1986. (in Chinese) [Google Scholar]
- Stpniewska, Z.; Wolinska, A.; Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009, 21, 1142–1147. [Google Scholar] [CrossRef]
- Liang, W.; Wu, Z.B.; Cheng, S.P.; Zhou, Q.H.; Hu, H.Y. Roles of substrate microorganisms and urease activities in wastewater purification in a constructed wetland system. Ecol. Eng. 2003, 21, 191–195. [Google Scholar] [CrossRef]
- Cheema, S.A.; Khan, M.I.; Tang, X.; Zhang, C.; Shen, C.; Malik, Z.; Ali, S.; Yang, J.; Shen, K.; Chen, X. Enhancement of phenanthrene and pyrene degradation in rhizosphere of tall fescue (Festuca arundinacea). J. Hazard. Mater. 2009, 166, 1226–1231. [Google Scholar] [CrossRef]
- Muyzer, G.; Ellen, C.W.; Andre, G.U. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar]
- Lin, H.R.; Chen, X.C.; Hu, S.P.; Shen, C.F.; Chen, G.C.; Shi, J.Y.; Chen, Y.X. Lead availability and soil microbial community composition in rice rhizosphere affected by thiosulfate addition. Appl. Soil. Ecol. 2010, 45, 232–237. [Google Scholar] [CrossRef]
- Zhang, J.; Hang, M.I.N. Eco-toxicity and metal contamination of paddy soil in an e-wastes recycling area. J. Hazard. Mater. 2009, 165, 744–750. [Google Scholar] [CrossRef]
- Shen, C.; Huang, S.; Wang, Z.; Qiao, M.; Tang, X.; Yu, C.; Shi, D.; Zhu, Y.; Shi, J.; Chen, X. Identification of Ah receptor agonists in soil of e-waste recycling sites from Taizhou area in China. Environ. Sci. Technol. 2007, 42, 49–55. [Google Scholar]
- Choi, S.D.; Baek, S.Y.; Chang, Y.S.; Wania, F.; Ikonomou, M.G.; Yoon, Y.J.; Park, B.K.; Hong, S. Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: Implications for long-range transport and local pollution. Environ. Sci. Technol. 2008, 42, 7125–7131. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Li, Y.F.; Wang, D.; Jia, H.; Harner, T.; Sverko, E.; Wan, X.; Xu, D.; Ren, N. Analysis of polychlorinated biphenyls in concurrently sampled Chinese air and surface soil. Environ. Sci. Technol. 2008, 42, 6514–6518. [Google Scholar] [CrossRef]
- Jiang, Q. Characteristics of PCB congeners and homologues in Chinese transformer oil. China Environ. Sci. 2007, 27, 608–612. [Google Scholar]
- Ren, N.; Que, M.; Li, Y.F.; Liu, Y.; Wan, X.; Xu, D.; Sverko, E.; Ma, J. Polychlorinated biphenyls in Chinese surface soils. Environ. Sci. Technol. 2007, 41, 3871–3876. [Google Scholar] [CrossRef]
- Akmal, M.; Wang, H.Z.; Wu, J.J.; Xu, J.M.; Xu, D.F. Changes in enzymes activity, substrate utilization pattern and diversity of soil microbial communities under cadmium pollution. J. Environ. Sci. China 2005, 17, 802–807. [Google Scholar]
- Hu, B.; Liang, D.; Liu, J.; Xie, J. Ecotoxicological effects of Cu and Se combined pollution on soil enzyme activities in planted and unplanted soil. Environ. Toxicol. Chem. 2013, 32, 1109–1116. [Google Scholar] [CrossRef]
- Speir, T.W.; Van Schaik, A.P.; Hunter, L.C.; Ryburn, J.L.; Percival, H.J. Attempts to derive EC50 values for heavy metals from land-applied Cu-, Ni-, and Zn-spiked sewage sludge. Soil Biol. Biochem. 2007, 39, 539–549. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, P.; Mao, L.; Zhi, Y.; Shi, W. Assessment of effects of heavy metals combined pollution on soil enzyme activities and microbial community structure: Modified ecological dose—Response model and PCR-RAPD. Environ. Earth Sci. 2010, 60, 603–612. [Google Scholar] [CrossRef]
- Andreoni, V.; Cavalca, L.; Rao, M.; Nocerino, G.; Bernasconi, S.; Dell’Amico, E.; Colombo, M.; Gianfreda, L. Bacterial communities and enzyme activities of PAHs polluted soils. Chemosphere 2004, 57, 401–412. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, W.; Wang, R.; Han, X.; Wang, Q. Microbial community diversity in the profle of an agricultural soil in northern China. J. Environ. Sci. 2008, 20, 981–988. [Google Scholar] [CrossRef]
- Huang, J.; Sheng, X.; He, L.; Huang, Z.; Wang, Q.; Zhang, Z. Characterization of depth-related changes in bacterial community compositions and functions of a paddy soil profile. FEMS Microbiol. Lett. 2013, 347, 33–42. [Google Scholar] [CrossRef]
- Smolders, E.; Buekers, J.; Oliver, I.; McLaughlin, M.J. Soil properties affecting toxicity of zinc to soil microbial properties in laboratory-spiked and field-contaminated soils. Environ. Toxicol. Chem. 2004, 23, 2633–2640. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tang, X.; Hashmi, M.Z.; Long, D.; Chen, L.; Khan, M.I.; Shen, C. Influence of Heavy Metals and PCBs Pollution on the Enzyme Activity and Microbial Community of Paddy Soils around an E-Waste Recycling Workshop. Int. J. Environ. Res. Public Health 2014, 11, 3118-3131. https://doi.org/10.3390/ijerph110303118
Tang X, Hashmi MZ, Long D, Chen L, Khan MI, Shen C. Influence of Heavy Metals and PCBs Pollution on the Enzyme Activity and Microbial Community of Paddy Soils around an E-Waste Recycling Workshop. International Journal of Environmental Research and Public Health. 2014; 11(3):3118-3131. https://doi.org/10.3390/ijerph110303118
Chicago/Turabian StyleTang, Xianjin, Muhammad Z. Hashmi, Dongyan Long, Litao Chen, Muhammad I. Khan, and Chaofeng Shen. 2014. "Influence of Heavy Metals and PCBs Pollution on the Enzyme Activity and Microbial Community of Paddy Soils around an E-Waste Recycling Workshop" International Journal of Environmental Research and Public Health 11, no. 3: 3118-3131. https://doi.org/10.3390/ijerph110303118






