Microbial Contamination Detection in Water Resources: Interest of Current Optical Methods, Trends and Needs in the Context of Climate Change
Abstract
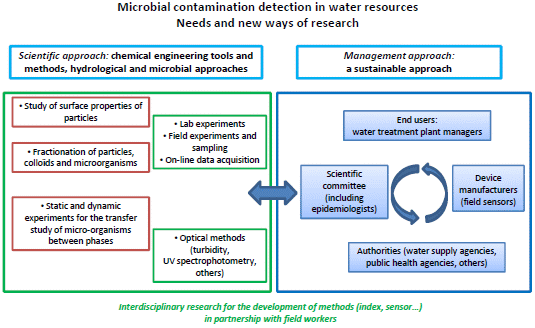
:1. Introduction
2. Sources, Fate and Behavior of Microorganisms in Water
2.1. Fate in Sediments and in Submerged Aquatic Vegetation
2.2. Transport and Fate of Microorganisms
2.3. Influence of Hydrometeorological Conditions

| Type of pathogens | Matrix | Water Body | References | Comments |
|---|---|---|---|---|
| Bacteria | Particles | Karstic aquifer | [67] | Connections with the surface responsible for turbid and bacterial contaminations |
| Bacteria groups | Colloids | Groundwater | [46] | Modelisation of transport mechanisms of the combined system (contaminant–colloids–bacteria) |
| Lakes | [47] | Correlation between size particles and transport and distribution after a storm | ||
| Recreational waters | [66] | Connection between microbial tracers and fecal indicator organisms | ||
| E. coli, Enterococci | Particles | Run-off | [68] | Rainfall simulations for erodible soil particles and sparsely vegetable soils |
| [45] | run-off | |||
| E. coli | Sediments | Rivers | [41] | Modelisation of bacteria transport during rainfall events |
| Virus Norovirus | Colloids | Rivers | [69] | Direct spillage of wastewater in river during heavy rains |
| Mixture | Colloids | Distributed water | [3] | Correlation of heavy rains with gastroenteritis epidemics |
| Particles | River, karstic water | [17] | Correlation of turbidity, flow rate and gastroenteritis epidemics | |
| Others Giardia cyst Protozoan parasites groups | Colloids | Rivers | [70] | Correlation with rainy events |
| Particles | Waterbeds soils | [52] | Interaction between parasites and particles (organic and inorganic) |
3. Optical Monitoring of Microbial Contamination: Current Methods, Trends and Needs
3.1. Current Trends: Turbidity, Particle Size Distribution (PSD) and Cytometry
3.2. Trends in Optical Methods
3.2.1. Fluorescence Measurements
3.2.2. Biosensors
3.2.3. Spectrophotometric Methods
| References | Optical domains | Measurement/study | Particle size (µm) | Suspended matter concentration (mg/L) |
|---|---|---|---|---|
| [98] | Visible and near-infrared spectral regions | Relationships between the concentration, composition and size of suspended particles | 2.72–460 | 0–90 |
| [106] | UV spectrophotometry and laser granulometry | Characterization of heterogeneous suspensions | 0.4–2 × 103 | 100–670 |
| [107] | Coupling UV-spectrophotometry and laser granulometry | Heterogeneous suspensions, quantitative approach (size and concentration) | 0.05–103 | 10–350 |
| [108] | UV spectrophotometry | Study of the impact of mechanical treatments on wastewater solids by UV spectrophotometry | 10−3–103 | 10–220 |
| [109] | UV spectrophotometry and laser granulometry | Study of UV–vis responses of mineral suspensions in water | 1–100 | 10–250 |
4. Conclusions
| Parameter/References | Kind of media/applications fields/pathogens | Influencing parameters for the studies/interferences | Particle size/Number of celldetected |
|---|---|---|---|
| Turbidity/[7]–[72,73,74,75,76,77,78,79,80] | Natural and wastewaters | Plankton, Humic substances | 10–103 µm |
| PSD/[3,84] | Karstic waters | Hydroclimatical | 0.9–1.5 µm |
| Cytometry/[85,86] | All fluorescent species | Others fluorescent species (e.g., humic-like substances) + light scattering | From virus to bacteria/107 colony forming unit/mL |
| Fluorescence, Bacteriophage life cycle/[87,88,89,90,91,92,93] | River waters (tyrosine, tryptophan and fulvic-like substances, E. Coli, Vibrio fischeri | Light-scattering, inner filters effects, bioluminescence interferences | From molecule to bacteria |
| Biosensors/[94,95,96] | Environment, food process, military | Interfering enzyme reactions | Virus to protozoan> 100 cells/mL |
| Spectrophotometry Methods/[97,98,99,100,101,102,103,104,105,106,107,108,109] | Virus, bacteria, cyanobacteria, nanoplanktonic and chlorophytes diatoms | Light scattering | 10−3–2 × 103 µm |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hlavsa, M.C.; Roberts, V.A.; Anderson, A.R.; Hill, V.R.; Kahler, A.M.; Orr, M.; Garrison, L.E.; Hicks, L.A.; Newton, A.; Hilborn, E.D.; et al. Surveillance for waterborne disease outbreaks and other health events associated with drinking water—United States, 2007–2008. MMWR Surveill. Summ. 2011, 60, 1–32. [Google Scholar]
- Auld, H.; MacIver, D.; Klaassen, J. Heavy rainfall and waterborne disease outbreaks: The Walkerton example. J. Toxicol. Environ. Health. A 2004, 67, 1879–1887. [Google Scholar]
- Curriero, F.C.; Patz, J.A.; Rose, J.B.; Lele, S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health 2001, 91, 1194–1199. [Google Scholar] [CrossRef]
- Hunter, P.R. Climate change and waterborne and vector-borne disease. J. Appl. Microbiol. 2003, 94, 37S–46S. [Google Scholar] [CrossRef]
- Bouzid, M.; Hooper, L.; Hunter, P.R. The effectiveness of public health interventions to reduce the health impact of climate change: A systematic review of systematic reviews. PLoS One 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, B.G.; Craun, G.F.; Yoder, J.S.; Hill, V.; Calderon, R.L.; Chen, N.; Lee, S.H.; Levy, D.A.; Beach, M.J. Surveillance for waterborne-disease outbreaks associated with drinking water—United States, 2001–2002. MMWR 2004, 53, 23–45. [Google Scholar]
- Pitkänen, T.; Karinen, P.; Miettinen, I.T.; Lettojärvi, H.; Heikkilä, A.; Maunula, R.; Aula, V.; Kuronen, H.; Vepsäläinen, A.; Nousiainen, L.-L.; et al. Microbial contamination of groundwater at small community water supplies in Finland. Ambio 2010, 40, 377–390. [Google Scholar]
- Risebro, H.L.; Breton, L.; Aird, H.; Hooper, A.; Hunter, P.R. Contaminated small drinking water supplies and risk of infectious intestinal disease: A prospective cohort study. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Rutter, M.; Nichols, G.L.; Swan, A.; De Louvois, J. A survey of the microbiological quality of private water supplies in England. Epidemiol. Infect. 2000, 124, 417–425. [Google Scholar] [CrossRef]
- Said, B.; Wright, F.; Nichols, G.L.; Reacher, M.; Rutter, M. Outbreaks of infectious disease associated with private drinking water supplies in England and Wales 1970–2000. Epidemiol. Infect. 2003, 130, 469–479. [Google Scholar]
- Kay, D.; Watkins, J.; Francis, C.A.; Wyn-Jones, A.P.; Stapleton, C.M.; Fewtrell, L.; Wyer, M.D.; Drury, D. The microbiological quality of seven large commercial private water supplies in the United Kingdom. J. Water Health 2007, 5, 523–538. [Google Scholar] [CrossRef]
- Bartram, J.; Corrales, L.; Davison, A.; Deere, D.; Drury, D.; Gordon, B.; Rinehold, A.; Stevens, M. Water Safety Plan Manual: Step-by-Step Risk Management for Drinking-Water Suppliers; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water. Technical Paper of the Intergovernmental Panel on Climate Change. IPCC Secretariat: Geneva, Switzerland, 2008; pp. 1–210. [Google Scholar]
- Hundesa, A.; Maluquer de Motes, C.; Bofill-Mas, S.; Binana-Gimenez, N.; Girones, R. Identification of human and animal adenoviruses and polyomaviruses for determination of sources of fecal contamination in the environment. Appl. Environ. Microbiol. 2006, 72, 7886–7893. [Google Scholar] [CrossRef]
- Poma, H.R.; Gutiérrez Cacciabue, D.; Garcé, B.; Gonzo, E.E.; Rajal, V.B. Towards a rational strategy for monitoring of microbiological quality of ambient waters. Sci. Total Environ. 2012, 433, 98–109. [Google Scholar] [CrossRef]
- Beaudeau, P.; Valdes, D.; Damien, M.; Stemfelet, M.; Seux, R. Natural and technical factors in faecal contamination incidents of drinking water in small distribution networks, France, 2003–2004: A geographical study. J. Water Health 2010, 8, 20–33. [Google Scholar] [CrossRef]
- Beaudeau, P.; Rambaud, L.; Galey, C.; le Tertre, A.; Zeghoun, A. Risque D’infections Sporadiques Lié à L’ingestion D’eau Du Robinet: L’émergence D’une Approche Epidémiologique. In Proceeding of Second National Congress Société Française Santé Environnement (SFSE), Paris, France, 14–15 December 2011.
- Badgley, B.D.; Nayak, B.S.; Harwood, V.J. The importance of sediment and submerged aquatic vegetation as potential habitats for persistent strains of Enterococci in a subtropical watershed. Water Res. 2010, 44, 5857–5866. [Google Scholar] [CrossRef]
- Meays, C.L.; Broersma, K.; Nordin, R.; Mazumder, A. Source tracking fecal bacteria in water: A critical review of current methods. J. Environ. Manag. 2004, 73, 71–79. [Google Scholar] [CrossRef]
- Blanch, A.R.; Belanche-Munoz, L.; Bonjoch, X.; Ebdon, J.; Gantzer, C.; Lucena, F. Tracking the origin of faecal pollution in surface water: An ongoing project within the European Union Research Programme. J. Water Health 2004, 2, 249–260. [Google Scholar]
- Field, K.G.; Samadpour, M. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007, 41, 3517–3538. [Google Scholar] [CrossRef]
- Gourmelon, M.; Caprais, M.P.; Mieszkin, S.; Marti, R.; Wéry, N.; Jardé, E.; Derrien, M.; Jadas-Hécart, A.; Communal, P.Y.; Jaffrezic, A.; et al. Development of microbial and chemical MST tools to identify the origin of the faecal pollution in bathing and shellfish harvesting waters in France. Water Res. 2010, 44, 4812–4824. [Google Scholar] [CrossRef] [Green Version]
- Furtula, V.; Osachoff, H.; Derksen, G.; Juahir, H.; Colodey, A.; Chambers, P. Inorganic nitrogen, sterols and bacterial source tracking as tools to characterize water quality and possible contamination sources in surface water. Water Res. 2012, 46, 1079–1092. [Google Scholar] [CrossRef]
- Mesquita, S.; Noble, R.T. Recent Developments in Monitoring of Microbiological Indicators of Water Quality across a Range of Water Types. In Water Resources Planning, Development and Management; Wurbs, R., Ed.; Texas A&M University: College Station, TX, USA, 2013; Chapter 2. [Google Scholar]
- Plummer, J.D.; Long, S.C. Monitoring source water for microbial contamination: Evaluation of water quality measures. Water Res. 2007, 41, 3716–3728. [Google Scholar] [CrossRef]
- Edge, T.A.; Hill, S.; Seto, P.; Marasalek, J. Library-dependent and library-independent microbial source tracking to identify spatial variation in faecal contamination sources along a lake Ontario beach (Ontario, Canada). Water Sci. Technol. 2010, 62, 719–727. [Google Scholar] [CrossRef]
- Kortbaoui, R.; Locas, A.; Imbeau, M.; Payment, P.; Villemur, R. Universal mitochondrial PCR combined with species-specific dot-blot assay as a source-tracking method of human, bovine, chicken, ovine, and porcine in fecal-contaminated surface water. Water Res. 2009, 43, 2002–2010. [Google Scholar] [CrossRef]
- Lyautey, E.; Lu, Z.; Lapen, D.R.; Berkers, T.E.; Edge, T.A.; Topp, E. Optimization and validation of rep-PCR genotypic libraries for microbial source tracking of environmental Escherichia coli isolates. Can. J. Microbiol. 2010, 56, 651–659. [Google Scholar] [CrossRef]
- Lee, D.Y.; Weir, S.C.; Lee, H.; Trevors, J.T. Quantitative identification of fecal water pollution sources by TaqMan real-time PCR assays using Bacteriodales 16S rRNA genetic markers. Appl. Microbiol. Biotechnol. 2010, 88, 1373–1383. [Google Scholar] [CrossRef]
- Staley, C.; Reckhow, K.H; Lukasik, J.; Harwood, V.J. Assessment of sources of human pathogens and fecal contamination in a Florida freshwater lake. Water Res. 2012, 46, 5799–5812. [Google Scholar] [CrossRef]
- Marsalek, J.; Rochfort, Q.J. Urban wet-weather flows: Sources of fecal contamination impacting on recreational waters and threatening drinking-water sources. J. Toxicol. Environ. Health A. 2004, 6, 1765–1777. [Google Scholar] [CrossRef]
- Selvakumar, A.; Borst, M.J. Variation of microorganism concentrations in urban stormwater runoff with land use and seasons. J. Water Health 2006, 4, 109–124. [Google Scholar]
- Dechesne, M.; Soyeux, E.; Loret, J.F.; Westrell, T.; Stenström, T.A.; Gornik, V.; Koch, C.; Exner, M.; Stanger, M.; Agutter, P.; et al. Pathogens in Source Water, Microbiological Risk Assessment: A Scientific Basis for Managing Drinking Water Safety from Source to Tap; Microrisk European Project: Nieuwegein, The Netherlands, 2006; pp. 1–42. [Google Scholar]
- Ferguson, C.M.; Charles, K.; Deere, D.D. Quantification of microbial sources in drinking-water catchments. Crit. Rev. Environ. Sci. Technol. 2008, 39, 1–40. [Google Scholar] [CrossRef]
- Harmel, R.D.; Karthikeyan Gentry, R.; Srinivasan, T.R. Effects of agricultural management, land use and watershed scale on E.coli concentrations in runoff and stream flow. Trans. ASABE 2010, 53, 1833–1841. [Google Scholar] [CrossRef]
- James, E.; Joyce, M. Assessment and management of watershed microbial contaminants. Crit. Rev. Environ. Sci. Technol. 2004, 34, 109–139. [Google Scholar] [CrossRef]
- George, I.; Anzil, A.; Servais, P. Quantification of fecal coliform inputs to aquatic systems through soil leaching. Water Res. 2004, 38, 611–618. [Google Scholar] [CrossRef]
- Gao, G.; Falconer, R.A.; Lin, B. Numerical modelling of sediment bacteria interaction processes in surface waters. Water Res. 2011, 45, 1951–1960. [Google Scholar] [CrossRef]
- Garzio-Hadzick, A.; Shelton, D.R.; Hill, R.L.; Pachepsky, Y.A.; Guber, A.K.; Rowland, R. Survival of manure-borne E. coli in streambed sediment: Effects of temperature and sediment properties. Water Res. 2010, 44, 2753–2762. [Google Scholar] [CrossRef]
- Chandran, A.; Varghese, S.; Kandeler, E.; Thomas, A.; Hatha, M.; Mazumder, A. An assessment of potential public health risk associated with the extended survival of indicator and pathogenic bacteria in freshwater lake sediments. Int. J. Hyg. Environ. Health 2011, 214, 258–264. [Google Scholar] [CrossRef]
- Cho, K.H.; Pachepsky, Y.A.; Kim, J.H.; Guber, A.K.; Shelton, D.R.; Rowland, R. Release of Escherichia coli from the bottom sediment in a first-order creek: Experiment and reach-specific modeling. J. Hydrol. 2010, 391, 322–332. [Google Scholar] [CrossRef]
- Jamieson, R.; Joy, D.M.; Lee, H.; Kostaschuk, R.; Gordon, R. Transport and deposition of sediment-associated Escherichia coli in natural streams. Water Res. 2005, 39, 2665–2675. [Google Scholar] [CrossRef]
- Muirhead, R.W.; Collins, R.P.; Bremer, P.J. Interaction of Escherichia coli and soil particles in runoff. Appl. Environ. Microbiol. 2006, 72, 3406–3411. [Google Scholar] [CrossRef]
- Loveland, J.P.; Ryan, J.N.; Amy, G.L.; Harvey, R.W. The reversibility of virus attachment to mineral surfaces. Colloids Surf. A 1996, 107, 205–221. [Google Scholar] [CrossRef]
- Soupir, M.L.; Mostaghimi, S. Escherichia coli and Enterococci attachment to particles in runoff from highly and sparsely vegetated grassland. Wat. Air Soil Poll. 2011, 216, 167–178. [Google Scholar] [CrossRef]
- Bekhit, H.M.; El-Hordy, M.A.; Hassan, A.E. Contaminant transport in groundwater in the presence of colloids and bacteria: Model development and verification. J. Contam. Hydrol. 2009, 108, 152–167. [Google Scholar] [CrossRef]
- Brookes, J.D.; Antenucci, J.; Hipsey, M.; Burch, M.D.; Ashbolt, N.J.; Fergusson, C. Fate and transport of pathogens in lakes and reservoirs. Environ. Int. 2004, 30, 741–759. [Google Scholar]
- Garcia-Armisen, T.; Servais, P. Partitioning and fate of particle-associated E. coli in river waters. Water Environ. Res. 2009, 81, 21–28. [Google Scholar]
- Gutierrez, L.; Nguyen, T.H. Interactions between rotavirus and Suwannee River organic matter: Aggregation, deposition, and adhesion force measurement. Environ. Sci. Technol. 2012, 21, 8705–8713. [Google Scholar] [CrossRef]
- Abudalo, R.A.; Ryan, J.N.; Harvey, R.W.; Metge, D.W.; Landkamer, L. Influence of organic matter on the transport of Cryptosporidium parvum oocysts in a ferric oxyhydroxide-coated quartz sand saturated porous medium. Water Res. 2010, 44, 1104–1113. [Google Scholar] [CrossRef]
- Searcy, K.E.; Packman, A.I.; Atwill, E.R.; Harter, T. Association of Cryptosporidium parvum with suspended particles: Impact on oocyst sedimentation. Appl. Environ. Microbiol. 2005, 71, 1072–1078. [Google Scholar] [CrossRef]
- Dumètre, A.; Aubert, D.; Puech, P.H.; Hohweyer, J.; Azas, N.; Villena, I. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbiol. 2012, 78, 905–912. [Google Scholar] [CrossRef]
- Auer, M.T; Niehaus, S.L. Modeling fecal-coliform bacteria. 1. Field and laboratory determination of loss kinetics. Water Res. 1993, 27, 693–701. [Google Scholar] [CrossRef]
- Ferguson, C.; Husman, A.M.D.; Altavilla, N.; Deere, D.; Ashbolt, N. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 2003, 33, 299–361. [Google Scholar] [CrossRef]
- Sinton, L.W.; Finlay, R.K.; Lynch, P.A. Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 1999, 65, 3605–3613. [Google Scholar]
- Noble, R.; Lee, I.; Schiff, K. Inactivation of indicator microorganisms from various sources of faecal contamination in seawater and freshwater. J. Appl. Microbiol. 2004, 96, 464–472. [Google Scholar] [CrossRef]
- Gronewold, A.D.; Myers, L.; Swall, J.L.; Noble, R.T. Addressing uncertainty in fecal indicator bacteria dark inactivation rates. Water Res. 2011, 45, 652–664. [Google Scholar] [CrossRef]
- Delpla, I.; Baures, E.; Jung, A.-V.; Thomas, O. Impacts of rainfall events on runoff water quality in an agricultural environment in temperate areas. Sci. Total Environ. 2011, 409, 1683–1688. [Google Scholar]
- Hata, A.; Katayama, H.; Kojima, K.; Sano, S.; Kasuga, I.; Kitajima, M.; Furumai, H. Effects of rainfall events on the occurrence and detection efficiency of viruses in river water impacted by combined sewer overflows. Sci. Total Environ. 2013, 468, 757–763. [Google Scholar]
- McBride, G.B.; Stott, R.; Miller, W.; Bambic, D.; Wuertz, S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Res. 2013, 47, 5282–5297. [Google Scholar] [CrossRef]
- Beaudeau, P. Impact Sanitaire D’un Accident Sur le Réseau D’adduction en eau Potable du Havre, D’une Panne De Désinfection à FECAMP et de 4 Episodes de Turbidité Dans Des Secteurs Ruraux (Seine-Maritime, 1998). Rapport de la Direction Départementale des Affaires Sanitaires et Sociales de Seine-Maritime et du Laboratoire D’études et D’analyses de la Ville du Havre; Report for the Social and Sanitary Departmental Direction (Seine-Maritime) and the studies and analyses Laboratory (Havre): Havre, MT, USA, 1999. [Google Scholar]
- Beaudeau, P.; de Valk, H.; Vaillant, V.; Mannschott, C.; Tillier, C.; Mouly, D.; Ledrans, M. Lessons learned from ten investigations of waterborne gastroenteritis outbreaks, France, 1998–2006. J. Water Health 2008, 6, 491–502. [Google Scholar] [CrossRef]
- Zmirou, D.; Ferley, J.P.; Collin, J.F.; Charrel, M.; Berlin, J. A follow-up study of gastro-intestinal diseases related to bacteriologically substandard drinking water. Am. J. Public Health 1987, 77, 582–584. [Google Scholar] [CrossRef]
- Brookes, J.D.; Hipsey, M.R.; Burch, M.D.; Regel, R.H.; Linden, L.G.; Ferguson, C.M.; Antenucci, J.P. Relative value of surrogate indicators for detecting pathogens in lakes and reservoirs. Environ. Sci. Technol. 2005, 39, 8614–8621. [Google Scholar] [CrossRef]
- Hipsey, M.R.; Antenucci, J.P.; Brookes, J.D.; Burch, M.D.; Regel, R.H.; Davies, C.M.; Ashbolt, N.J.; Ferguson, C. Hydrodynamic of Pathogens in Lakes and Reservoirs; American Water Works Research Foundation: Denver, CO, USA, 2005; Report 91073F. [Google Scholar]
- Wyer, M.D.; Kay, D.; Watkins, J.; Davies, C.; Kay, C.; Thomas, R.; Porter, J.; Stapleton, C.M.; Moore, H. Evaluating short-term changes in recreational water quality during a hydrograph event using a combination of microbial tracers, environmental microbiology, microbial source tracking and hydrological techniques: A case study in Southwest Wales, UK. Water Res. 2011, 44, 4783–4795. [Google Scholar]
- Gargala, G. Evaluation Des Risques Humains et Environnementaux Associés à La Présence de Cryptosporidium Dans L’environnement Hydrique de Haute-Normandie. In Proceeding of Second National Congress Société Française Santé Environnement (SFSE), Paris, France, 14–15 December 2011.
- Soupir, M.L.; Mostaghimi, S.; Dillaha, T. Attachment of Escherichia coli and Enterococci to particles in runoff. J. Environ. Qual. 2010, 39, 1019–1027. [Google Scholar] [CrossRef]
- Tillaut, H.; Encrenaz, N.; Checlair, E.; Alexandre-Bird, A.; Gomes Do Esperito Santo, E.; Beaudeau, P. Epidémie de gastro-entérite, Isère, novembre 2002. Bull. Environ. Hydrol. 2004, 12, 47–48. [Google Scholar]
- Delbec, M.; Chesnot, T.; Mignard, C.; Duchemin, J. Risques Microbiologiques Emergents Pour la Ressource en Eau, Cas de L’agglomération Parisienne. In Proceeding of Second National Congress Société Française Santé Environnement (SFSE), Paris, France, 14–15 December 2011.
- Beaudeau, P.; Pascal, M.; Mouly, D.; Galey, C.; Thomas, O. Health risks associated with drinking water in a context of climate change in France: A review of surveillance requirements. J. Water Clim. Change 2011, 2, 230–246. [Google Scholar] [CrossRef]
- Aumond, M.; Joannis, C. Turbidity Monitoring in Sewage. In Proceeding of 10th International Conference on Urban Drainage, Copenhague, Denmark, 21–26 August 2005.
- Henckens, G.; Veldkamp, R.; Schuit, T. On Monitoring of Turbidity in Sewers. Global Solutions for Urban Drainage. In Proceedings of the Ninth International Conference on Urban Drainage (9ICUD), Portland, OR, USA, 8–13 September 2002.
- Langeveld, J.G.; Veldkamp, R.G.; Clemens, F. Suspended solids transport: An analysis based on turbidity measurements and event based fully calibrated hydrodynamic models. Water Sci. Technol. 2005, 52, 93–101. [Google Scholar]
- Ruban, G.; Bertrand-Krajewski, J.L.; Chebbo, G.; Gromaire, M.C.; Joannis, C. Accuracy and reproducibility of turbidity measurements in urban waste water. Houille Blanche. Revue Internationale de l'Eau 2006, 4, 129–135. [Google Scholar]
- Chebbo, G.; Bachoc, A.; Laplace, D.; Leguennec, B. The transfer of solids in combined sewer networks. Water Sci. Technol. 1995, 31, 95–105. [Google Scholar]
- Deletic, A.B.; Maksimovic, C.T. Evaluation of water quality factors in storm runoff from paved areas. J. Environ. Eng. 1998, 124, 869–879. [Google Scholar] [CrossRef]
- Maréchal, A. Relations Entre Caractéristiques de la Pollution Particulaire et Paramètres Optiques Dans Les Eaux Résiduaires Urbaines. PhD Thesis, Institut national polytechnique de Lorraine, Nancy, France, 2000. [Google Scholar]
- Grüning, H.; Orth, H. Investigations of the dynamic behaviour of the composition of combined sewage using on-line analyzers. Water Sci. Technol. 2002, 45, 77–83. [Google Scholar]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Dynamics and interaction of organic carbon, turbidity and bacteria in a Karst aquifer system. Hydrogeol. J. 2006, 14, 473–484. [Google Scholar] [CrossRef]
- Page, R.M.; Scheidler, S.; Polat, E.; Svoboda, P.; Huggenberger, P. Faecal indicator bacteria: Groundwater dynamics and transport following precipitation and river water infiltration. Water Air Soil Pollut. 2012, 223, 2771–2782. [Google Scholar] [CrossRef]
- Hannouche, A.; Chebbo, G.; Ruban, G.; Tassin, B.; Joannis, C. Relation entre la turbidité et les matières en suspension en réseau d’assainissement unitaire. Techniques Sciences et Méthodes 2011, 10, 42–50. [Google Scholar]
- Goldscheider, N.; Pronk, M.; Zopfi, J. New insights into the transport of sediments and microorganisms in Karst groundwater by continuous monitoring of particle-size distribution. Geol. Croat. 2010, 63, 137–142. [Google Scholar]
- Atteia, O.; Kozel, R. Particle size distributions in waters from a Karstic aquifer: From particles to colloids. J. Hydrol. 1997, 201, 102–119. [Google Scholar] [CrossRef]
- Ferrari, B.C.; Stoner, K.; Bergquist, P.L. Applying fluorescence based technology to the recovery and isolation of Cryptosporidium and Giardia from Industrial wastewater streams. Water Res. 2006, 40, 541–548. [Google Scholar] [CrossRef]
- King, D.N.; Brenner, K.P.; Rodgers, M.R. A critical evaluation of a flow cytometer used for detecting Enterococci in recreational waters. J. Water Health 2007, 5, 295–306. [Google Scholar]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Naden, P.S.; Old, G.H.; Eliot-Laize, C.; Granger, S.J.; Hawkins, J.M.B.; Bol, R.; Haygarth, P. Assessment of natural fluorescence as a tracer of diffuse agricultural pollution from slurry spreading in intensively-farmed grasslands. Water Res. 2010, 44, 1701–1712. [Google Scholar]
- Jaffrezic, A.; Jardé, E.; Pourcher, A.M.; Gourmelon, M.; Caprais, M.P.; Heddadj, D.; Cottinet, P.; Bilal, M.; Derrien, M.; Marti, R.; et al. Microbial and chemical markers: Runoff transfer in animal manure-amended soils. J. Environ. Qual. 2011, 40, 959–968. [Google Scholar] [CrossRef]
- Lefcourt, A.M.; Kim, M.S.; Chen, Y.-R. A transportable fluorescence imagining system for detecting fecal contaminants. Comput. Electron. Agr. 2005, 48, 63–74. [Google Scholar] [CrossRef]
- Shahid, P.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar]
- Birmele, M.; Ripp, S.; Jegier, P.; Roberts, M.S.; Sayler, G; Garland, J.L. Characterization and validation of a bioluminescent bioreporter for the direct detection of Escherichia coli. J. Microbiol. Methods 2008, 75, 354–356. [Google Scholar] [CrossRef]
- Connelly, J.T.; Baeumner, A.J. Biosensors for the detection of waterborne pathogens. Anal. Bioanal. Chem. 2012, 402, 117–127. [Google Scholar] [CrossRef]
- Xu, X.; Ying, Y. Microbial biosensors for environmental monitoring and food analysis. Food Rev. Int. 2011, 27, 300–329. [Google Scholar] [CrossRef]
- Pérez-Lopez, B.; Merkoçi, A. Portable Chemical Sensors: Weapons against Bioterrorism. In Biosensors for Safety and Security Applications; Nikolelis, D.P., Ed.; Springer: Berlin, Germany, 2012; pp. 43–61. [Google Scholar]
- Bowers, D.G.; Binding, C.E. The optical properties of mineral suspended particles: A review and synthesis. Estuarine Coastal Shelf Sci. 2006, 67, 1–2. [Google Scholar] [CrossRef]
- Astoreca, R.; Doxaran, D.; Ruddick, K.; Rousseau, V.; Lancelot, C. Influence of suspended particle concentration, composition and size on the variability of inherent optical properties of the Southern North Sea. Continental Shelf Res. 2012, 35, 117–128. [Google Scholar] [CrossRef]
- Stramski, D.; Mobley, C.D. Effects of microbial particles on oceanic optics: A database of single-particle optical properties. Limnol. Oceanography 1997, 42, 538–549. [Google Scholar] [CrossRef]
- Stadler, H.; Klock, E.; Skritek, P.; Mach, R.L.; Zerobin, W.; Farnleitner, A.H. The spectral absorption coefficient at 254nm as a real-time early warning proxy for detecting faecal pollution events at alpine karst water resources. Wat. Sci. Technol. 2010, 62, 1898–1906. [Google Scholar] [CrossRef]
- Thomas, O.; Burgess, C. From Spectra to Qualitative and Quantitative Results. In UV-Visible Spectrophotometry of Water and Wastewater; Thomas, O., Burgess, C., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–360. [Google Scholar]
- Thomas, O.; Mazas, N.; Massiani, C. Determination of biodegradable dissolved organic carbon in waters with the use of UV absorptiometry. Environ. Technol. 1993, 14, 487–493. [Google Scholar] [CrossRef]
- Thomas, O.; El Khorassani, H.; Touraud, E.; Bitar, H. TOC vs. UV spectrophotometry for wastewater quality monitoring. Talanta 1999, 50, 743–749. [Google Scholar] [CrossRef]
- Thomas, O.; Gallot, S. UV multiwavelength absorptiometry (UVMA) for the examination of natural waters and wastewaters. Fresenius J. Anal. Chem. 1990, 338, 234–237. [Google Scholar] [CrossRef]
- Thomas, O.; Theraulaz, F.; Domeizel, M.; Massiani, C. UV spectral deconvolution: A valuable tool for wastewater quality determination. Environ. Technol. 1993, 14, 1187–1192. [Google Scholar] [CrossRef]
- Azema, N.; Pouet, M.-F.; Berho, C.; Thomas, O. Wastewater suspended solids study by optical methods. Colloids Surf. A 2002, 204, 131–140. [Google Scholar] [CrossRef]
- Bayle, S.; Azéma, N.; Berho, C.; Pouet, M.-F.; Lopez-Cuesta, J.-M.; Thomas, O. Study of heterogeneous suspensions: A new quantitative approach coupling laser granulometry and UV-visible spectrophotometry. Colloids Surf. A 2005, 262, 242–250. [Google Scholar] [CrossRef]
- Berho, C.; Pouet, M.-F.; Thomas, O. Study of the impact of mechanical treatments on waste water solids by UV spectrophotometry. Environ. Technol. 2003, 24, 1545–1551. [Google Scholar] [CrossRef]
- Berho, C.; Pouet, M.-F.; Bayle, S.; Azema, N.; Thomas, O. Study of UV-visible responses of mineral suspensions in water. Colloids Surf. A 2004, 248, 9–16. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jung, A.-V.; Le Cann, P.; Roig, B.; Thomas, O.; Baurès, E.; Thomas, M.-F. Microbial Contamination Detection in Water Resources: Interest of Current Optical Methods, Trends and Needs in the Context of Climate Change. Int. J. Environ. Res. Public Health 2014, 11, 4292-4310. https://doi.org/10.3390/ijerph110404292
Jung A-V, Le Cann P, Roig B, Thomas O, Baurès E, Thomas M-F. Microbial Contamination Detection in Water Resources: Interest of Current Optical Methods, Trends and Needs in the Context of Climate Change. International Journal of Environmental Research and Public Health. 2014; 11(4):4292-4310. https://doi.org/10.3390/ijerph110404292
Chicago/Turabian StyleJung, Aude-Valérie, Pierre Le Cann, Benoit Roig, Olivier Thomas, Estelle Baurès, and Marie-Florence Thomas. 2014. "Microbial Contamination Detection in Water Resources: Interest of Current Optical Methods, Trends and Needs in the Context of Climate Change" International Journal of Environmental Research and Public Health 11, no. 4: 4292-4310. https://doi.org/10.3390/ijerph110404292
APA StyleJung, A.-V., Le Cann, P., Roig, B., Thomas, O., Baurès, E., & Thomas, M.-F. (2014). Microbial Contamination Detection in Water Resources: Interest of Current Optical Methods, Trends and Needs in the Context of Climate Change. International Journal of Environmental Research and Public Health, 11(4), 4292-4310. https://doi.org/10.3390/ijerph110404292