Assessment of the Microbiological Quality of Groundwater in Three Regions of the Valencian Community (Spain)
Abstract
:1. Introduction
2. Experimental Section
2.1. Test Site Characteristics
2.2. Groundwater Bodies (GWB)
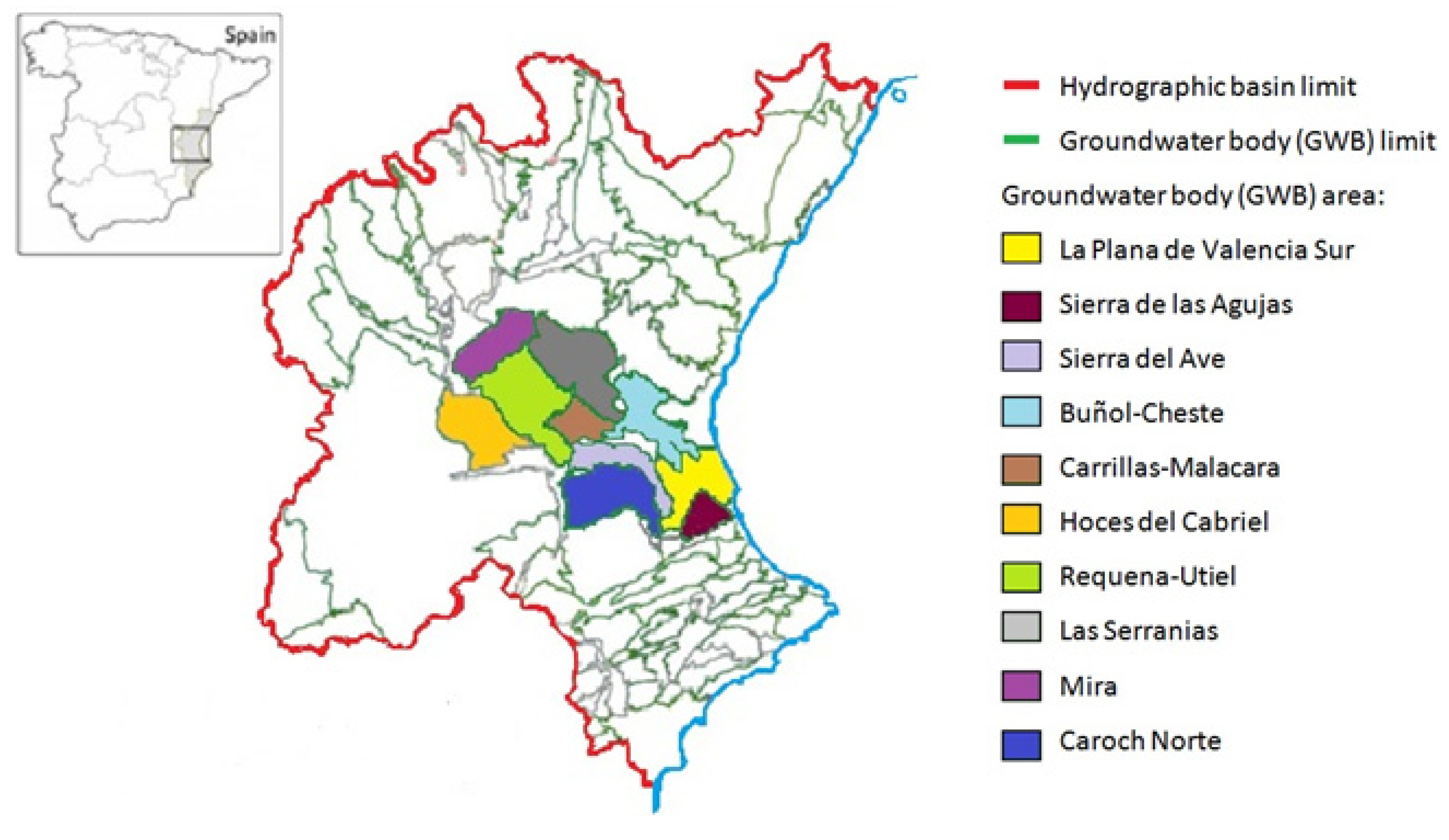
2.3. Database
2.4.Data Grouping
2.5. Statistical Analysis
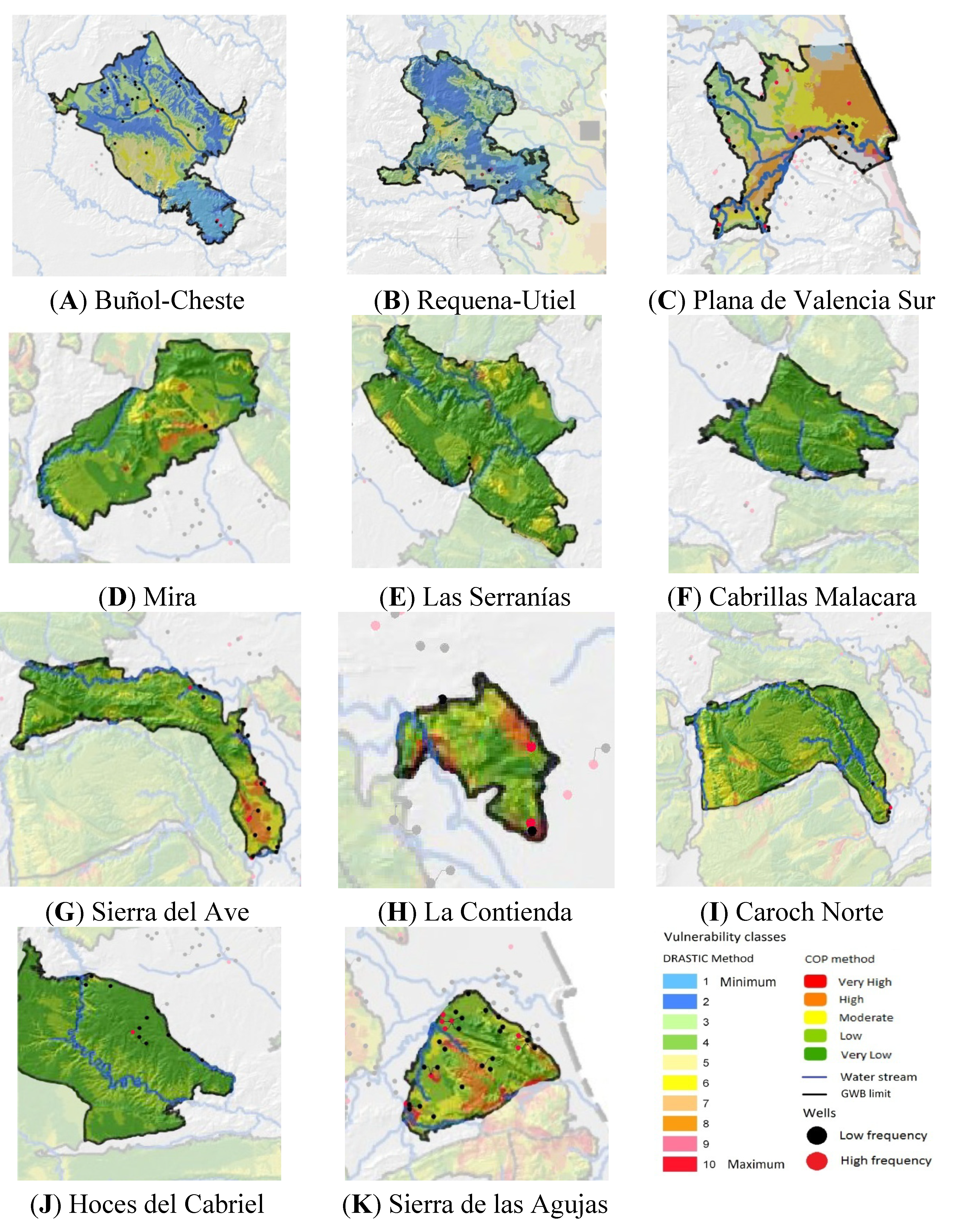
2.6. Vulnerability Maps
3. Results
| Categories | Criteria | No. Wells | Percentage (%) | Region | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Utiel Requena | Ribera Alta | Ribera Baja | p | |||||||
| No. Wells | % | No. Wells | % | No. Wells | % | |||||
| <1 | Low frequency | 113 | 81.88 | 47 | 92.16 | 46 | 73.02 | 20 | 83.33 | 0.030 |
| 1–100 | High frequency | 25 | 18.12 | 4 | 7.84 | 17 | 26.98 | 4 | 16.67 | 0.030 |
| All wells | 138 | 100 | 51 | 36.96 | 63 | 45.65 | 24 | 17.39 | <0.001 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Categories | No. samples | No. Wells | E. coli | Percentage of positive wells | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | FE | SRC | |||
| 1 | 258 | 113 | 0.12 | 1.99 | 0.00 | 32.00 | 11.5 | 6.19 |
| 1–100 | 60 | 25 | 4.02 | 10.00 | 0.00 | 55.00 | 64 | 36 |
| All wells | 318 | 138 | -- | -- | -- | -- | 21.01 | 11.59 |
| p | -- | -- | -- | -- | -- | -- | <0.001 | <0.001 |
| Criteria (Well) | Categories | Statistical analysis of difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–100 | ||||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | ||
| Depth (m) | 140.83 | 108.37 | 0.00 | 560.00 | 126.04 | 87.40 | 4.00 | 300.00 | t = 0.62; p = 0.472 |
| Static level (m) | 48.79 | 63.66 | 0.00 | 400.00 | 39.75 | 33.69 | 0.00 | 120.00 | t = −0.95; p = 0.343 |
| Flow (L/min) | 2,272.29 | 2,490.08 | 0.00 | 14,166.00 | 3,102.08 | 3,590.89 | 0.00 | 15,000.00 | t = 1.10; p = 0.281 |
| Proximity to population centr (m) | 1,572.29 | 2,064.99 | 0.00 | 10,000.00 | 1,300.0 | 1,750.70 | 0.00 | 5,000.00 | t = −0.69; p = 0.492 |
| Categories | GWB type and vulnerability class | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonated * | Detrital-Mixed * | ||||||||||
| No. wells | Very high | High | Moderate | Low | Very Low | No. wells | High (8,9,10) | Intermediate (5,6,7) | Low (3,4) | VeryLow (1,2) | |
| <1 | 51 | 9.8 | 25.49 | 29.41 | 9.8 | 25.49 | 62 | 9.67 | 40.32 | 40.32 | 9.67 |
| 1–100 | 14 | 21.42 | 35.71 | 0 | 35.71 | 7.14 | 11 | 0 | 63.63 | 9.09 | 27.27 |
| All wells | 65 | -- | -- | -- | -- | -- | 73 | -- | -- | -- | -- |
| Risk factors of Contamination * | Risk factors (expressed as percentage) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cased (type of well) | Fenced | Shed | Distance < 90 m | ||||||
| Solid waste landfill | Cesspool | Sewer | Wastewater | Farms | Irrigation | ||||
| Yes | 24.00 | 36 | 40 | 84 | 56 | 24 | |||
| No | 76.00 | 64 | 52 | 88 | 4 | 32 | 88 | 100 | 76 |
| p | <0.001 | 0.048 | 0.392 | <0.001 | 0.087 | <0.001 | |||
4. Discussion
4.1. Microbiological Contamination
4.2.Spatial Distribution of Wells
4.3.Risk Factors
4.4. Type of Groundwater Body (GWB)
4.5.Maps and Classes of Vulnerability to Pollution
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- COST Action 620. Vulnerability and Risk Mapping for the Protection of Carbonated (Karst) Aquifers: Final Report. Available online: http://bookshop.europa.eu/is-bin/INTERSHOP.enfinity/WFS/EU-Bookshop-Site/en_GB/-/EUR/ViewPublication-Start?PublicationKey=KINA20912 (accessed on 20 May 2014).
- Vías, J.M.; Andreo, B.; Ravbar, N.; Hötzl, H. Mapping the vulnerability of groundwater to the contamination of four carbonated aquifers in Europe. J. Environ. Manag. 2010, 91, 1500–1510. [Google Scholar] [CrossRef]
- Aller, L.; Bennett, T.; Lehr, J.H.; Petty, R.J.; Hackett, G. Drastic: A Standardised System for Evaluating Groundwater Pollution Potential Using Hydrogeologic Setting; US Environment Protection Agency: Ada, OK, USA, 1987. [Google Scholar]
- Instituto Nacional de Estadística (INE). Available online: http://www.ine.es/FichasWeb/RegMunicipios.do?fichas=4&inputBusqueda=&comunidades=9006&botonFichas=Ir+a+la+tabla+de+resultados (accessed on 6 August 2013).
- MMA, P.S.A. Estudio Inicial Para la Identificación y Caracterización de las Masas de Agua Subterránea de las Cuencas Intercomunitarias (No. Tomo I), Memorias y Anexos 1,2 y 3; Ministerio de Medio Ambiente: Madrid, Spain, 2005. [Google Scholar]
- Ferguson, A.S.; Layton, A.C.; Mailloux, B.J.; Culligan, P.J.; Williams, D.E.; Smartt, A.E.; Sayler, G.S.; Feighery, J.; McKay, L.D.; Knappett, P.S.K.; et al. Comparison of fecal indicators with pathogenic bacteria and rotavirus in groundwater. Sci. Total Environ. 2012, 431, 314–322. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, Third Edition, Incorporating First and Second Addenda 2008. Available online: http://www.who.int/water_sanitation_health/dwq/gdwq3rev/en/ (accessed on 17 July 2013).
- CDC—Epi InfoTM. Available online: http://wwwn.cdc.gov/epiinfo/index.htm (accessed on 8 June 2013).
- Vías, J.M.; Andreo, B.; Perles, M.J.; Carrasco, F.; Vadillo, I.; Jiménez, P. Preliminary Proposal of a Method for Contamination Vulnerability Mapping in Carbonated Aquifers. In Karst and Environment; Carrasco, F., Durán, J., Andreo, B., Eds.; Fundacion Cueva de Nerja: Málaga, Spain, 2002; pp. 75–83. [Google Scholar]
- Martinez, C.; Jiménez, A.; Gómez, J.D.; Díaz, J.A.; Luque, J.A.; Moreno, L.R.; Morales, R. Actividad 9: Protección de las Aguas Subterráneas Empleadas Para Consumo Humano Según los Requerimientos de la Directiva Marco del Agua; Demarcación Hidrográfica del Júcar: Madrid, Spain, 2009. [Google Scholar]
- Li, R.; Merchant, J.W. Modeling vulnerability of groundwater to pollution under future scenarios of climate change and biofuels-related land use change: A case study in North Dakota, USA. Sci. Total Environ. 2013, 447, 32–45. [Google Scholar] [CrossRef]
- Huan, H.; Wang, J.; Teng, Y. Assessment and validation of groundwater vulnerability to nitrate based on a modified DRASTIC model: A case study in Jilin City of northeast China. Sci. Total Environ. 2012, 440, 14–23. [Google Scholar] [CrossRef]
- Howard, G.; Pedley, S.; Barrett, M.; Nalubega, M.; Johal, K. Risk factors contributing to microbiological contamination of shallow groundwater in Kampala, Uganda. Water Res. 2003, 37, 3421–3429. [Google Scholar] [CrossRef]
- Conboy, M.; Goss, M. Natural protection of groundwater against bacteria of fecal origin. J. Contam. Hydrol. 2000, 43, 1–24. [Google Scholar] [CrossRef]
- Powell, K.L.; Taylor, R.G.; Cronin, A.A.; Barrett, M.H.; Pedley, S.; Sellwood, J.; Trowsdale, S.A.; Lerner, D.N. Microbial contamination of two urban sandstone aquifers in the UK. Water Res. 2003, 37, 339–352. [Google Scholar] [CrossRef]
- Foppen, J.W.A.; van Herwerden, M.; Kebtie, M.; Noman, A.; Schijven, J.F.; Stuyfzand, P.J.; Uhlenbrook, S. Transport of Escherichia coli and solutes during waste water infiltration in an urban alluvial aquifer. J. Contam. Hydrol. 2008, 95, 1–16. [Google Scholar] [CrossRef]
- Cho, J.C.; Cho, H.B.; Kim, S.J. Heavy contamination of a subsurface aquifer and a stream by livestock wastewater in a stock farming area, Wonju, Korea. Environ. Pollut. 2000, 109, 137–146. [Google Scholar] [CrossRef]
- Rice, E.W.; Covert, T.C.; Wild, D.K.; Berman, D.; Johnson, S.A.; Johnson, C.H. Comparative resistance of escherichia coli and enterococci to chlorination. J. Environ. Sci. Health Part A 1993, 28, 89–97. [Google Scholar]
- Van Geen, A.; Ahmed, K.M.; Akita, Y.; Alam, M.J.; Culligan, P.J.; Emch, M.; Escamilla, V.; Feighery, J.; Ferguson, A.S.; Knappett, P.; et al. Fecal contamination of shallow tubewells in bangladesh inversely related to arsenic. Environ. Sci. Technol. 2011, 45, 1199–1205. [Google Scholar] [CrossRef]
- Li, B.L.; Loehle, C.; Malon, D. Microbial transport through heterogeneous porous media: Random walk, fractal, and percolation approaches. Ecol. Model. 1996, 85, 285–302. [Google Scholar] [CrossRef]
- Taylor, R.; Cronin, A.; Pedley, S.; Barker, J.; Atkinson, T. The implications of groundwater velocity variations on microbial transport and wellhead protection—Review of field evidence. FEMS Microbiol. Ecol. 2004, 49, 17–26. [Google Scholar] [CrossRef]
- Abdelkader, B.; Abdelhak, M.; Abdeslam, K.; Ahmed, M.; Brahim, Z. Estimation of pollution load of domestic sewage to oued bechar (SW Algeria) and its impact on the microbiological quality of groundwater. Procedia. Eng. 2012, 33, 261–267. [Google Scholar] [CrossRef]
- Verstraeten, I.M.; Fetterman, G.S; Meyer, M.T.; Bullen, T.; Sebree, S.K. Use of tracers and isotopes to evaluate vulnerability of water in domestic wells to septic waste. Ground Water Monit. Remediat. 2005, 25, 107–117. [Google Scholar] [CrossRef]
- Andreo, B.; Goldscheider, N.; Vadillo, I.; Vías, J.M.; Neukum, C.; Sinreich, M.; Jiménez, P.; Brechenmacher, J.; Carrasco, F.; Hötzl, H.; et al. Karst groundwater protection: First application of a Pan-European Approach to vulnerability, hazard and risk mapping in the Sierra de Líbar (Southern Spain). Sci. Total Environ. 2006, 357, 54–73. [Google Scholar] [CrossRef]
- Edmonds, C. Improved groundwater vulnerability mapping for the karstic chalk aquifer of south east England. Eng. Geol. 2008, 99, 95–108. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Llopis-González, A.; Sánchez, A.L.; Requena, P.M.; Suárez-Varela, M.M. Assessment of the Microbiological Quality of Groundwater in Three Regions of the Valencian Community (Spain). Int. J. Environ. Res. Public Health 2014, 11, 5527-5540. https://doi.org/10.3390/ijerph110505527
Llopis-González A, Sánchez AL, Requena PM, Suárez-Varela MM. Assessment of the Microbiological Quality of Groundwater in Three Regions of the Valencian Community (Spain). International Journal of Environmental Research and Public Health. 2014; 11(5):5527-5540. https://doi.org/10.3390/ijerph110505527
Chicago/Turabian StyleLlopis-González, Agustín, Adriana L. Sánchez, Pedro Martí Requena, and María Morales Suárez-Varela. 2014. "Assessment of the Microbiological Quality of Groundwater in Three Regions of the Valencian Community (Spain)" International Journal of Environmental Research and Public Health 11, no. 5: 5527-5540. https://doi.org/10.3390/ijerph110505527





