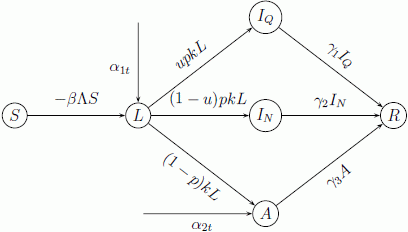
3.1. Antiviral Drugs Prophylaxis and Isolation Strategy
Because effective vaccines will take about six months to produce once a novel influenza virus has been confirmed, the use of antiviral drugs is one of the most important intervention measures in the case of a pandemic [
4,
23]. We first consider that control strategy only via antiviral drugs for prophylaxis during an outbreak of pandemic influenza.
Figure 2.
(
a) The plot of the control reproduction number
Rc as a function of doses
m, where
u = 0 and other parameters assume the values of
Table 1; (
b) the plot for which
Rc is equal to one. Every parameter point (
m,
u) that corresponds to
Rc < 1 lies above this curve and
Rc > 1 for those points that lie below the
Rc = 1 curve.
Figure 2.
(
a) The plot of the control reproduction number
Rc as a function of doses
m, where
u = 0 and other parameters assume the values of
Table 1; (
b) the plot for which
Rc is equal to one. Every parameter point (
m,
u) that corresponds to
Rc < 1 lies above this curve and
Rc > 1 for those points that lie below the
Rc = 1 curve.
Figure 2a shows the plot of the control reproduction number
Rc as a function of doses
m, where
u = 0 and other parameters suppose the values of
Table 1. We see that the control reproduction number
Rc remains greater than one, even for substantial values of doses
m. If
Rc is above one, the disease will widely spread in the population; hence, the successful containment of the epidemic is to reduce the reproduction number below one. It is impossible to contain the epidemic only by the use of the antiviral drugs if antiviral drugs have low-efficacy in reducing infectiousness against a new virus strain. Therefore, it is very necessary to implement other control measures in conjunction with the use of antiviral drugs.
Isolation is one of the most effective methods to contain the spread of an outbreak [
27]. For our purposes, we consider that the control strategy is taking the isolation strategy in conjunction with the use of antiviral drugs for prophylaxis. There are two possibilities when taking antiviral prophylaxis and the isolation strategy:
R0 >
Rc > 1 and
R0 > 1 >
Rc. The first corresponds to failure containment, and infected arrivals could lead to an outbreak. In this paper, we focus on the second scenario,
i.e.,
Rc can be reduced to below one when taking antiviral prophylaxis and the isolation strategy widely and effectively.
Figure 2b shows the values of
m and
u for which the control reproduction number
Rc is equal to one, namely
Rc = 1 for every parameter point (
m,
u) that lies on the curve. Meanwhile, every parameter point (
m,
u) that corresponds to
Rc < 1 lies above this curve and
Rc > 1 for those points that lie below this curve. By comparing the two curves in
Figure 2, we can see that the reproduction number that is greater than one can be reduced to below one by the implementation of isolation of cases. Suppose the stockpile of antiviral drugs is sufficient; at least 20% of the infected individuals need to be isolated for reducing the reproduction number below one. On the other hand, at least 60% of the infected individuals need to be isolated if no antiviral drugs are provided.
To provide a more intuitive result,
Figure 3 shows plots of the cumulative percentage of infected individuals up to time t and the percentage of isolated infected individuals
versus time t, respectively, as predicted by the model (1) with the parameter values of the model listed in
Table 1, for different proportions of
u, where
u = 0, 0.3, 0.5, 0.7, 0.9 and
m = 15. Comparing the curves in
Figure 3a, we can see that taking antiviral prophylaxis combined with the isolation strategy can reduce substantially the cumulative number of infected individuals. At 180 days, the cumulative number of infected individuals when
u = 0.3 drops to 1/25 of the cumulative number of infected individuals when no cases are isolated. By way of illustration, for a city with one million population members, allowing for one imported infective individual and one imported exposed individual per day, the cumulative number of infected individuals will be less than 2, 134 and the number of daily isolated cases will be less than six for a duration of six months if half of the symptomatic cases can be isolated.
Figure 3.
(
a) Plots of the cumulative percentage of infected individuals up to time
t; and (
b) plots of the percentage of isolated cases
versus time
t, as predicted by the model (1) with parameters:
R0 = 1.5,
a = 0.6,
b = 0.2,
m = 15 and other parameters supposing the values of
Table 1, for different values of
u, where
u = 0, 0.3, 0.5, 0.7, 0.9.
Figure 3.
(
a) Plots of the cumulative percentage of infected individuals up to time
t; and (
b) plots of the percentage of isolated cases
versus time
t, as predicted by the model (1) with parameters:
R0 = 1.5,
a = 0.6,
b = 0.2,
m = 15 and other parameters supposing the values of
Table 1, for different values of
u, where
u = 0, 0.3, 0.5, 0.7, 0.9.
In addition, from
Figure 3a, we can see that the cumulative percentage of infected individuals is always a monotonically decreasing function of
u,
i.e., increasing the proportion of isolation can reduce the number of infected individuals; but the percentage of isolated infected individuals is only a monotonically decreasing function of
u, after some fixed time point. This can be interpreted as follows. With the doses
m fixed,
Rc decreases when the proportion
u of isolation increases. Hence, in a short time horizon, the percentage of the isolated cases increases as
u increases; but in a long time horizon, as
Rc decreases, the percentage of cumulative infected individuals decreases, and so does the percentage of the isolated cases. In conclusion, for containing the epidemic better, we need to keep
Rc under a level as low as possible, which implies that it is important to isolate infected individuals as much as possible in the early stage of the disease.
From
Figure 3b, we can see that as long as a few infected individuals need to be isolated every day and the reproductive number is reduced to below one, then the total number of the infected individuals can be reduced greatly (see
Figure 3a).
It is obvious from the above that implementing an isolation strategy has an obvious effect on the containment of the epidemic, especially if antiviral drugs have low-efficacy in reducing infectiousness.
3.2. Comparisons between the Approximate Values and the Actual Values of the Percentage of Cumulative Symptomatic Cases, the Percentage of Cumulative Isolated Infected Individuals and the Intervention Cost
Figure 4a,b shows the actual values and the approximate values of the cumulative percentages of symptomatic cases and isolated cases as functions of time
t when
Rc = 0.8. Comparing the dashed lines with the solid lines in
Figure 4, we can find that the dashed lines are located above the solid lines all of the time, but there is little difference between them, and they have the same change trends. This illustrates that the difference between the actual values and the approximate values is very little. More specifically, for a duration of 180 days, for every million individuals, the absolute error of the cumulative number of symptomatic individuals is 129, and the absolute error of the cumulative number of isolated cases is 80. Thus, in practice, we can take the approximate values as the estimations of the actual values, and the errors are quite small. On the other hand, the expressions of estimated values are simple and easy to be calculated. Based on the above merits, the approximate method is very useful for the management department to make control strategies. However, it should be noted that the absolute errors of the cumulative percentages of symptomatic individuals and isolated cases increase with the control reproduction number
Rc increasing. In other words, the smaller the control reproduction number
Rc is, the more accurate the approximation is. As we can see in
Figure 4c, the curve begins relatively flat and after a while becomes very steep when
Rc exceeds 0.9. This implies that the approximation precision is relatively high if the reproduction number can be reduced below 0.9, and the approximation precision is significantly lowered when
Rc is over 0.9. The low accuracy may be caused by the approximation of
S ≈ 1. The deviation between the percentage of susceptible individuals and one cannot be ignored when
Rc is close to one.
Figure 4.
(a,b) Plots of the actual values and the approximate values of the cumulative percentages of infected individuals and isolated cases as functions of time t, respectively, where Rc = 0.8, m = 15 and u =0.62; (c) plots of the absolute errors of the cumulative percentage of symptomatic cases as a function of Rc; (d) plots of the absolute errors of the cumulative percentage of isolated cases as a function of Rc.
Figure 4.
(a,b) Plots of the actual values and the approximate values of the cumulative percentages of infected individuals and isolated cases as functions of time t, respectively, where Rc = 0.8, m = 15 and u =0.62; (c) plots of the absolute errors of the cumulative percentage of symptomatic cases as a function of Rc; (d) plots of the absolute errors of the cumulative percentage of isolated cases as a function of Rc.
Figure 5 shows the actual intervention cost and the approximate intervention cost as given by the model (1) with
c1 = 1,
c2 = 50,
c3 = 100,
c4 = 100 and other parameters supposing the values of
Table 1. The three graphs all show that the difference between the actual intervention cost and the approximate intervention cost is very little. The reason is that the approximate values of the cumulative percentage of symptomatic cases and the cumulative percentage of isolated cases are very close to their actual values, and the cost is a linear combination of them, so the result is inevitable.
At the same time, it is of interest to note that in
Figure 5b, the actual intervention cost is not necessarily a monotonically increasing function of doses m, and there is a turning point in the actual intervention cost curve, where it is minimum. A similar situation takes place in the approximate intervention cost curve. For all parameter values, the actual intervention cost and the approximate intervention cost show a trend downward at first, when they reach their bottoms, and then begin to rise again, as
m increases. This phenomenon tells us that in order to minimize the cost of intervention measures, a modest number of doses is enough, and too lager values of doses of antiviral drugs to reduce transmission only waste doses.
Figure 5.
(a) Plots of the actual intervention cost and the approximate intervention cost as functions of time t, where Rc =0.8, and the cost coefficients c1 = 1, c2 = 50, c3 = 100 and c4 = 100; (b) plots of the actual intervention cost and the approximate intervention cost as functions of doses m, where Rc = 0.8 and the parameters as (a); (c) plots of the actual intervention cost and the approximate intervention cost as functions of u, where m = 15 and the parameters as (a).
Figure 5.
(a) Plots of the actual intervention cost and the approximate intervention cost as functions of time t, where Rc =0.8, and the cost coefficients c1 = 1, c2 = 50, c3 = 100 and c4 = 100; (b) plots of the actual intervention cost and the approximate intervention cost as functions of doses m, where Rc = 0.8 and the parameters as (a); (c) plots of the actual intervention cost and the approximate intervention cost as functions of u, where m = 15 and the parameters as (a).
These results have obvious implications for a public health service system. First, we give an estimation of the number of the cumulative isolated infected, so that the hospital can prepare the resources (such as beds and personnel) for the possible epidemic in advance. Second, the estimation of the required number of antiviral drugs provides a reference for the management department about the antiviral stockpile size for controlling the epidemic. This is very significant for the management department, because a small stockpile of antiviral drugs cannot satisfy the demand of the containment epidemic, whereas, a very large stockpile of antiviral drugs is a great waste of medical resources. Finally, the estimation of the cost of intervention will help the government with financial preparation prior to the possible pandemic influenza.
3.3. Sensitivity Analysis
The baseline values of the parameters in
Table 1 may not be suitable to a newly emerged strain of influenza virus. Therefore, we carry out a sensitivity analysis to investigate the effect of varying these parameters, such as the basic reproduction number (
R0), the infectivity reduction factor for the isolated individuals (parameter
ε), the proportion of symptomatic cases (parameter
p), the relative infectivity of asymptomatic cases (parameter
σ), the imported rate of exposed individuals (parameter
α1), the imported rate of asymptomatic cases (parameter
α2), the efficacy of antiviral drugs (parameters
a and
b), the infectious period (parameters 1/
γ1, 1/
γ2, 1/
γ3) and the coefficients of the intervention cost (parameters
c1,
c2,
c3 and
c4).
Figures 6,
7,
8,
9,
10,
11,
12,
13 show their impact on the results in
Sections 3.1 and
3.2. When conducting the sensitivity analysis for a parameter, we assume the values of other parameters as
Table 1, unless indicated otherwise.
Figure 6.
(a) Plots of the cumulative percentages of infected individuals and isolated cases during 180 days, as functions of R0, where m = 15, u = 0.9; (b) plots for which Rc is equal to one for different values of R0, where R0 = 1.4, 1.5, 1.7, 2.0. Every parameter point (m, u) that corresponds to Rc < 1 lies above this curve and Rc > 1 for those points that lie below the Rc = 1 curve.
Figure 6.
(a) Plots of the cumulative percentages of infected individuals and isolated cases during 180 days, as functions of R0, where m = 15, u = 0.9; (b) plots for which Rc is equal to one for different values of R0, where R0 = 1.4, 1.5, 1.7, 2.0. Every parameter point (m, u) that corresponds to Rc < 1 lies above this curve and Rc > 1 for those points that lie below the Rc = 1 curve.
The effect of the basic reproduction number: Earlier in the manuscript, we supposed that the basic reproduction number
R0 is 1.5.
Figure 6a shows the effect of changing this assumption on the cumulative percentage of infected individuals. The cumulative percentage of infected individuals is quite sensitive to this change. For fixed parameters
m and
u, the cumulative percentage of infected individuals increases as
R0 increases, and it increases rapidly when
R0 surpasses some value. This is because the reproduction number cannot be reduced to below one for those values of
R0 that exceed some value. The change of
R0 gives the same impact on the cumulative percentage of isolated cases.
Figure 6b shows plots for which
Rc is equal to one for different values of
R0, where
R0 = 1.4, 1.5, 1.7, 2.0. Every parameter point (
m,
u) that satisfies
Rc < 1 lies above the corresponding curve. The increase in the reproduction number
R0 makes
Rc = 1 curve upward and shrinks significantly the set of parameter points for which
Rc < 1. This implies that it needs more effort to contain the transmission with larger
R0. Moreover, the containment of the epidemic will not succeed if the reproduction number is too high, such as
R0 > 2.5.
Figure 7.
The effect of the infectivity reduction factor for isolated individuals. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of ε, for a duration of six months, where m = 15, u = 0.9; (b) plots for which Rc = 1, for various values of ε, where ε = 1/6, 1/3, 1/2, 2/3.
Figure 7.
The effect of the infectivity reduction factor for isolated individuals. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of ε, for a duration of six months, where m = 15, u = 0.9; (b) plots for which Rc = 1, for various values of ε, where ε = 1/6, 1/3, 1/2, 2/3.
The effect of the infectivity reduction factor for the isolated individual: The above results assume that the infectivity reduction factor (parameter
ε) for the isolated individuals equals 1/6. The impact of altering this assumption is shown in
Figure 7. The change of
ε has a significant impact on the cumulative percentages of infected individuals and isolated cases (see
Figure 7a). The low value of f results in a much less cumulative percentages of infected individuals and isolated cases, while larger f generates much more infected individuals and isolated cases. Especially, the slopes of the two curves suddenly grow larger when the value of
ε exceeds 0.8, which means that the effectiveness of isolation strategies decreases significantly for
ε > 0.8. This implies that the effectiveness of the isolation strategies is very sensitive to the parameter
ε. Since the earlier the isolation begins, the better the effectiveness of the control strategies is, isolation strategies should begin as early as possible for better effectiveness of interventions. To obtain successful containment of the epidemic, the objective of the implementation of control measures is to bring
Rc below one.
Figure 7b shows the curves for which
Rc is equal to one for different values of
ε = 1/6, 1/3, 1/2, 2/3. As the parameter
ε increases, the set of scenarios for which containment is achievable becomes smaller and smaller. Even containment will fail if the parameter
ε is larger than some value.
The effect of the proportion of symptomatic cases:
Figure 8 shows the effect of varying the proportion of symptomatic cases (parameter
p). The effectiveness of control measures is very sensitive to the proportion of symptomatic cases. For the fixed parameters
m and
u, the cumulative percentage of infected individuals decreases substantially with the proportion of symptomatic cases increasing. This is because more infected individuals are treated/isolated and more susceptible individuals receive antiviral prophylaxis. In
Figure 8b, the set of scenarios for which containment is successful shrinks significantly as the proportion of symptomatic cases declines. If the proportion of symptomatic cases falls below a certain value (for example
p < 0.2), the epidemic is hard to control.
Figure 8.
The effect of the proportion of symptomatic cases. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of p, for a duration of six months, where m = 15, u = 0.9; (b) plots for which Rc = 1, for various values of parameter p, where p = 0.25, 0.35, 0.5, 0.75.
Figure 8.
The effect of the proportion of symptomatic cases. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of p, for a duration of six months, where m = 15, u = 0.9; (b) plots for which Rc = 1, for various values of parameter p, where p = 0.25, 0.35, 0.5, 0.75.
The effect of the relative infectivity of asymptomatic cases: The relative infectivity of asymptomatic cases (parameter
σ) is studied in the range 0.003–0.8. The impact of varying the parameter
σ on the cumulative percentages of infected individuals and isolated cases is shown in
Figure 9a. Unsurprisingly, the effectiveness of control measures declines as the infectivity of asymptomatic cases increases. A low infectivity of asymptomatic cases (
σ = 0.003) corresponds to a smaller cumulative number of infected individuals; conversely, a high infectivity (such as
σ = 0.8) generates more infected cases. However on the whole, the effect caused by the change of
σ is not very significant, since the two curves in
Figure 9a always gently rise.
Figure 9b shows the plots for which
Rc = 1, for different values of parameter
σ, where
σ = 0.003, 0.3, 0.5, 0.8. The set of scenarios for which containment is available becomes smaller as the values of parameter
σ grows larger, and the reproduction number can be reduced below one for all the values of
σ ranging from zero to one.
Figure 9.
The effect of the relative infectivity of asymptomatic cases. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of the parameter σ, for a duration of six months, where u = 0.9, m = 15; (b) plots for which Rc = 1, for various values of parameter σ, where σ = 0.003, 0.3, 0.5, 0.8.
Figure 9.
The effect of the relative infectivity of asymptomatic cases. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of the parameter σ, for a duration of six months, where u = 0.9, m = 15; (b) plots for which Rc = 1, for various values of parameter σ, where σ = 0.003, 0.3, 0.5, 0.8.
The effect of the imported rates of exposed individuals and asymptomatic cases: We use three values for the imported rates of exposed individuals and asymptomatic cases (
αi = 10
−7, 10
−6, 10
−5,
i = 1, 2) to illustrate the effects of varying the assumed values of them. The change of the imported rate of exposed individuals makes no difference to the control reproduction number
Rc, according to the expression of
Rc (Formula (4)). However, this change has an impact on the cumulative percentages of infected individuals and isolated cases. It should be noted that for a fixed value of
α2, the cumulative percentages of infected individuals and isolated cases increase linearly with the increase in the imported rate of exposed individuals (
α1). From
Figure 10b, we can see that the effect of varying the imported rate of asymptomatic cases (
α2) is similar to that of varying the parameter
α1.
Figure 10.
The effect of the imported rates of exposed individuals and asymptomatic cases. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of α1, for a duration of six months, where m = 15, u = 0.9; (b) plots of the cumulative percentages of infected individuals and isolated cases as functions of α2, for a duration of six months, where m = 15, u = 0.9.
Figure 10.
The effect of the imported rates of exposed individuals and asymptomatic cases. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of α1, for a duration of six months, where m = 15, u = 0.9; (b) plots of the cumulative percentages of infected individuals and isolated cases as functions of α2, for a duration of six months, where m = 15, u = 0.9.
The effect of the efficacy of antiviral drugs: In
Section 2.1, we assume that the effectiveness of dispensing
m antiviral doses per case is to reduce the transmission rate from
β to
βfm, where
fm =
a + (1 −
a)exp(−
bm). The sensitivity of
b has been discussed in the literature [
14]. For large doses m, the effectiveness of antiviral drugs in reducing transmission largely depends on the parameter
a. Therefore, we only consider the effect of varying the parameter
a. The impact of the change of
a on the cumulative percentages of infected individuals and isolated cases is shown in
Figure 11. The cumulative percentages of infected individuals and isolated cases increase as the effectiveness of antiviral drugs declines for the fixed values of m and
u. It is interesting to note that the influence of the change of
a on the cumulative percentages of infected individuals and isolated cases becomes smaller with the proportion of isolation increasing.
Figure 11d shows the curves for which
Rc = 1, for various values of parameter
a = 0.3, 0.5, 0.6, 0.8. The set of scenarios for which containment is successful becomes large with the parameter
a decreasing. In other words, containment is more likely to be successful when the value of
a is small. Unfortunately, there is no good data to estimate the antiviral efficacy for a new pandemic strain before a control policy is instituted. In this case, regardless of whether antiviral drugs can effectively mitigate the transmission of a new pandemic strain, non-pharmaceutical measures, such as case isolation, combined with antiviral drugs, can be used to reduce the spread of influenza strain.
Figure 11.
The effect of varying the efficacy of antiviral drugs. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of the parameter a, where m = 15 and u = 0.5; (b) same as (a), but for m = 15 and u = 0.7; (c) same as (a), but for m = 15 and u =0.9; (d) plots for which Rc = 1, for various values of parameter a, where a = 0.3, 0.5, 0.6, 0.8.
Figure 11.
The effect of varying the efficacy of antiviral drugs. (a) Plots of the cumulative percentages of infected individuals and isolated cases as functions of the parameter a, where m = 15 and u = 0.5; (b) same as (a), but for m = 15 and u = 0.7; (c) same as (a), but for m = 15 and u =0.9; (d) plots for which Rc = 1, for various values of parameter a, where a = 0.3, 0.5, 0.6, 0.8.
The effect of the infectious period: The above results are based on the assumption that the infectious period is 1.5 days [
17,
21]. However, some models suppose that the infectious period is four days [
3,
14]. We carry out sensitivity analysis on the value of the infectious period. The impact of varying infectious periods on the effectiveness of control strategies is shown in
Figure 12. The cumulative percentage of infected individuals is a little sensitive to the change of the infectious period with
ε = 1/6, but it is very sensitive to the change of the infectious period when
ε = 2/3 (see
Figure 12a). The cumulative percentage of isolated individuals has the similar result as the cumulative percentage of infected individuals.
Figure 12c,d shows the curves for which
Rc = 1 for different infectious periods 1/
γi, (
i = 1, 2, 3) with
ε = 1/6 and
ε= 2/3, respectively, where 1/
γi = 1.5, 2.5, 3.5, 4.5 (
i = 1, 2, 3).
The set of scenarios for which containment is successful expands with the infectious period extending, and the expansion is more obvious for a larger value of parameter ε.
Figure 12.
The effect of varying parameters determining infectious period. (a) Plots of the cumulative percentage of infected individuals as functions of the infectious period, for ε= 1/6, 1/3, 2/3, respectively; (b) same as (a), but for the cumulative percentage of isolated individuals; (c) plots for which Rc = 1 for different infectious period 1/γi, (i = 1, 2, 3) with ε= 1/6, where 1/γi = 1.5, 2.5, 3.5, 4.5; (d) same as (c), but with ε = 2/3.
Figure 12.
The effect of varying parameters determining infectious period. (a) Plots of the cumulative percentage of infected individuals as functions of the infectious period, for ε= 1/6, 1/3, 2/3, respectively; (b) same as (a), but for the cumulative percentage of isolated individuals; (c) plots for which Rc = 1 for different infectious period 1/γi, (i = 1, 2, 3) with ε= 1/6, where 1/γi = 1.5, 2.5, 3.5, 4.5; (d) same as (c), but with ε = 2/3.
The effect of the coefficients of the intervention cost: In order to make a sensitivity analysis on the coefficients of the intervention cost (parameters
c1,
c2) by comparing the actual cost with the approximate cost, we vary the coefficients
c1 and
c2, respectively.
Figure 13 shows the actual intervention cost and the approximate intervention cost as functions of time t using four different coefficients (
c1 = 1,
c2 = 50;
c1 = 1,
c2 = 200;
c1 = 50,
c2 = 1;
c1 = 200,
c2 = 1, respectively). The two cost curves in every graph are quite close to each other. This illustrates that the changes of
c1 and
c2 could not have a great influence on the difference between the actual intervention cost and the approximate intervention cost. Obviously, the changes of
c3 and
c4 have little impact on the conclusion that there is little difference between the actual intervention cost and the approximate intervention cost.
Figure 13.
Plots of the actual intervention cost and the approximate intervention cost versus time t for various values of c1 and c2, where c1 = 1, 50, 200, c2 = 1, 50, 200, Rc = 0.8, c3 = 100 and c4 = 100.
Figure 13.
Plots of the actual intervention cost and the approximate intervention cost versus time t for various values of c1 and c2, where c1 = 1, 50, 200, c2 = 1, 50, 200, Rc = 0.8, c3 = 100 and c4 = 100.