Meta-Analysis of the Copper, Zinc, and Cadmium Absorption Capacities of Aquatic Plants in Heavy Metal-Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Documentation Indexing
2.2. Statistical Analysis
| Experimental Site | pH | Family | Heavy Metal Concentration (mg·L−1) | Study | |||
|---|---|---|---|---|---|---|---|
| Cu | Zn | Cd | References | ||||
| 1 | Shenzhen Special Economic Zone, China | 3.65 | Rhizophoraceae | - | 7.26 | 0.77 | [7] |
| 2 | Part of Unnao city, U. P., India | 7.30 | Typhaceae, Cyperaceae | 2.61 | 2.79 | 0.96 | [8] |
| 3 | Zhejiang Province, China | - | Araceae, Cyperaceae, Gramineae, Iridaceae, Juncaceae, Lythraceae, Pontederiaceae, Typhaceae | 0.05 | 7.72 | - | [9] |
| 4 | West Bengal, India | 8.75 | Convolvulaceae, Compositae, Marsileaceae | 0.09 | 0.15 | 0.07 | [10] |
| 5 | Southern Assam, India | - | Amaranthaceae, Araceae, Athyriaceae, Chenopodiaceae, Compositae, Convolvulaceae, Cyperaceae, Euphorbiaceae, Labtatae, Leguminosae, Onagraceae, Pontederiaceae, Solanaceae, Umbelliferae | - | 1.48 | - | [11] |
| 6 | Northeast of Nantes, France | - | Juncaceae, Typhaceae | 0.25 | 2.00 | 0.10 | [12] |
| 7 | Northwest of Lake Taihu, China | 7.62 | Gramineae, Onagraceae | 0.74 | 2.59 | 0.12 | [13] |
| 8 | River Olobok, Poland | 7.00 | Hydrocharitaceae, Potamogetonaceae | 1.91 | - | 0.22 | [14] |
| River Pilawa, Poland | 6.60 | Hydrocharitaceae, Potamogetonaceae | 4.87 | - | 0.75 | [14] | |
| 9 | Southern Jiangsu Province, China | 7.30 | Ceratophyllaceae | 0.89 | 9.10 | 0.12 | [15] |
| 10 | Olesno, Poland | - | Typhaceae | 7.26 | 5.10 | - | [16] |
| 11 | South Bohemia, CzechRepublic | - | Gramineae, Typhaceae | 0.68 | 4.96 | 0.02 | [17] |
| 12 | Šalek Valley, Slovenia | 12.0 | Najadaceae, Potamogetonaceae | 1.20 | 2.00 | - | [18] |
| 13 | Lucknow, U. P., India | 6.48 | Asclepiadaceae, Chenopodiaceae, Compositae, Cyperaceae, Malvaceae, Solanaceae, Euphorbiaceae | 4.25 | - | 0.02 | [19] |
| 14 | Barra do Pira´s, Brazil | 6.80 | Leguminosae, Araceae, Pontederiaceae | 1.89 | 3.38 | 0.26 | [20] |
| 15 | Sohag City, Egypt | 7.6 | Ceratophyllaceae, Pontederiaceae, Haloragidaceae, Gramineae, Typhaceae | 0.03 | 0.11 | 0.01 | [21] |
2.3. Publication Bias
3. Results and Discussion
3.1. BCF for Copper, Zinc, and Cadmium


3.2. Relationship among Copper, Zinc, and Cadmium


3.3. Influencing Factors
3.3.1. Plant Organs
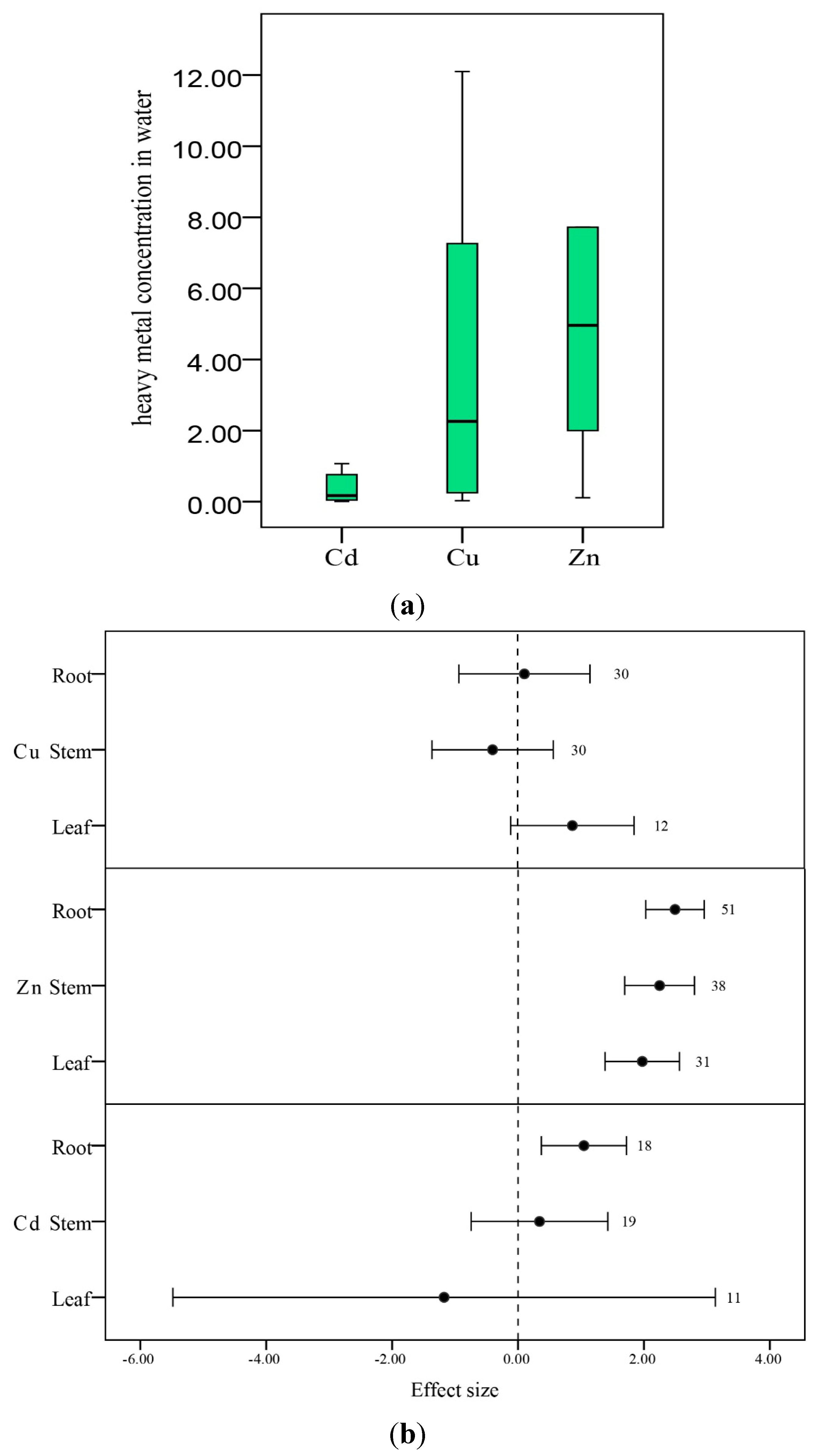
3.3.2. pH

3.3.3. Submerged and Emerged Plant Species

3.3.4. Soil, Water, and Plants
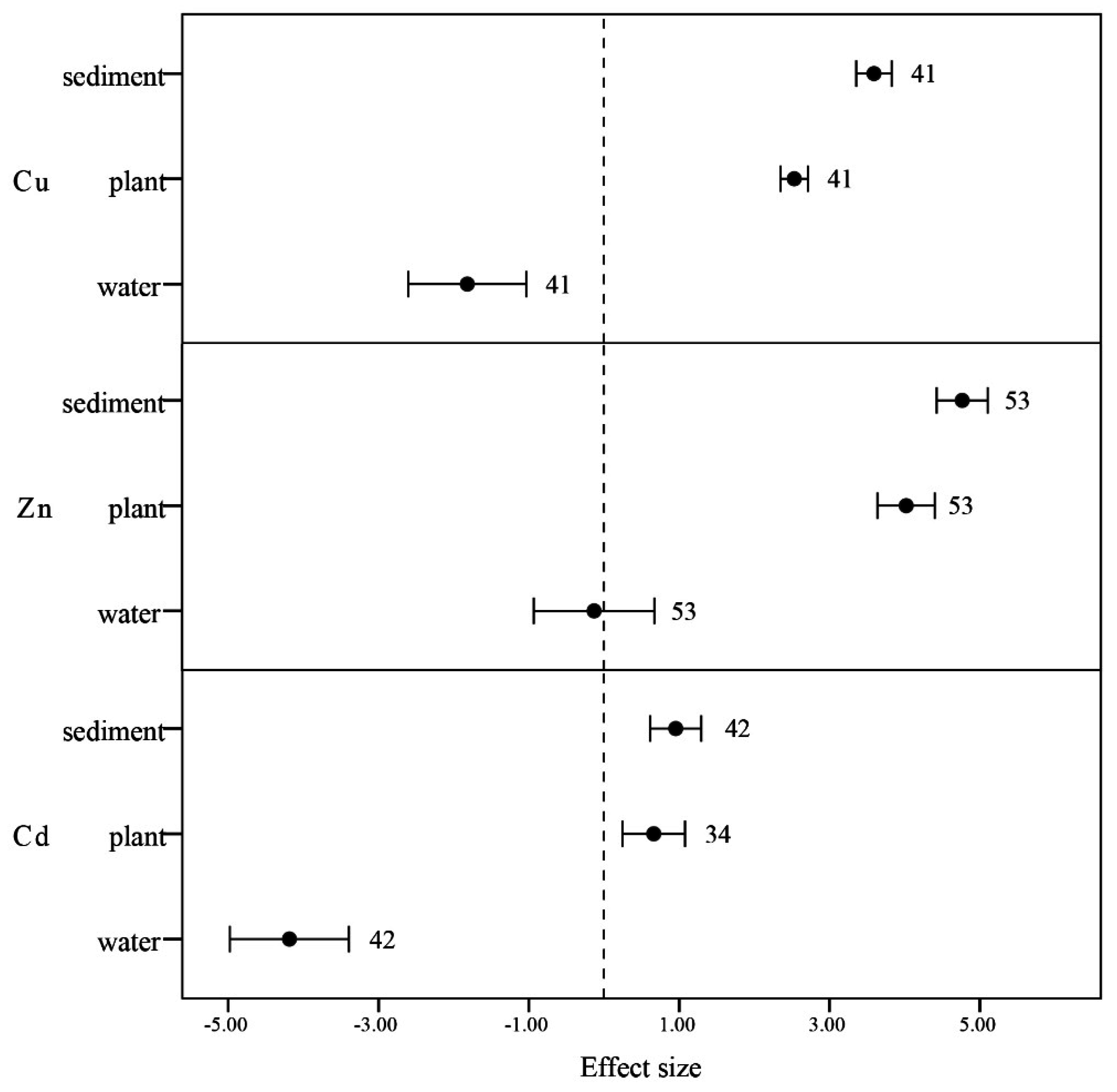
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldantoni, D.; Alfani, A.; Di Tommasi, P.; Bartoli, G.; De Santo, A.V. Assessment of Macro and Microelement Accumulation Capability of Two Aquatic Plants. Environ. Pollut. 2004, 130, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J. Accumulation of Cadmium in Crop Plants and its Consequences to Human Health. Adv. Agron. 1993, 51, 173–212. [Google Scholar]
- Jackson, L.J. Paradigms of Metal Accumulation in Rooted Aquatic Vascular Plants. Sci. Total Environ. 1998, 219, 223–231. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Effects of Chromium on Activated Sludge and On the Performance of Wastewater Treatment Plants: A Review. Water Res. 2012, 46, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Guittonny-Philippe, A.; Masotti, V.; H Hener, P.; Boudenne, J.; Viglione, J.; Laffont-Schwob, I. Constructed Wetlands to Reduce Metal Pollution From Industrial Catchments in Aquatic Mediterranean Ecosystems: A Review to Overcome Obstacles and Suggest Potential Solutions. Environ. Int. 2014, 64, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and Metalloid Removal in Constructed Wetlands, with Emphasis on the Importance of Plants and Standardized Measurements: A Review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.F.Y. Accumulation and Distribution of Heavy Metals in a Simulated Mangrove System Treated with Sewage. Hydrobiologia 1997, 352, 67–75. [Google Scholar] [CrossRef]
- Yadav, S.; Chandra, R. Heavy Metals Accumulation and Ecophysiological Effect on Typha angustifolia L. And Cyperus esculentus L. Growing in Distillery and Tannery Effluent Polluted Natural Wetland Site, Unnao, India. Environ. Earth Sci. 2011, 62, 1235–1243. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Z.; Gao, P.; Liu, P. Selection of Aquatic Plants for Phytoremediation of Heavy Metal in Electroplate Wastewater. Acta Physiol. Plant 2013, 35, 355–364. [Google Scholar] [CrossRef]
- Gupta, S.; Nayek, S.; Saha, R.N.; Satpati, S. Assessment of Heavy Metal Accumulation in Macrophyte, Agricultural Soil, and Crop Plants Adjacent to Discharge Zone of Sponge Iron Factory. Environ. Geol. 2008, 55, 731–739. [Google Scholar] [CrossRef]
- Mazumdar, K.; Das, S. Phytoremediation of Pb, Zn, Fe, and Mg with 25 Wetland Plant Species from a Paper Mill Contaminated Site in North East India. Environ. Sci. Pollut. Res. 2015, 22, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Ladislas, S.; El-Mufleh, A.; Gérente, C.; Chazarenc, F.; Andrès, Y.; Béchet, B. Potential of Aquatic Macrophytes as Bioindicators of Heavy Metal Pollution in Urban Stormwater Runoff. Water Air Soil Pollut. 2012, 223, 877–888. [Google Scholar] [CrossRef]
- Bo, L.; Wang, D.; Li, T.; Li, Y.; Zhang, G.; Wang, C.; Zhang, S. Accumulation and Risk Assessment of Heavy Metals in Water, Sediments, and Aquatic Organisms in Rural Rivers in the Taihu Lake Region, China. Environ. Sci. Pollut. Res. 2015, 22, 6721–6731. [Google Scholar] [CrossRef] [PubMed]
- Samecka-Cymerman, A.; Kempers, A.J. Heavy Metals in Aquatic Macrophytes From Two Small Rivers Polluted by Urban, Agricultural and Textile Industry Sewages Sw Poland. Arch. Environ. Contam. Toxicol. 2007, 53, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shen, Z.; Zhu, S.; Wang, W. Heavy Metals in Wetland Plants and Soil of Lake Taihu, China. Environ. Toxicol. Chem. 2008, 27, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Klink, A.; Macioł, A.; Wisłocka, M.; Krawczyk, J. Metal Accumulation and Distribution in the Organs of Typha Latifolia L. (Cattail) and their Potential Use in Bioindication. Limnologica 2013, 43, 164–168. [Google Scholar] [CrossRef]
- Březinová, T.; Vymazal, J. Evaluation of Heavy Metals Seasonal Accumulation in Phalaris Arundinacea in a Constructed Treatment Wetland. Ecol. Eng. 2015, 79, 94–99. [Google Scholar] [CrossRef]
- Mazej, Z.; Germ, M. Trace Element Accumulation and Distribution in Four Aquatic Macrophytes. Chemosphere 2009, 74, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Bauddh, K.; Kumar, S.; Dwivedi, N.; Singh, D.P.; Barman, S.C. Accumulation of Metals in Weed Species Grown On the Soil Contaminated with Industrial Waste and their Phytoremediation Potential. Ecol. Eng. 2013, 61, 491–495. [Google Scholar] [CrossRef]
- Valitutto, R.S.; Sella, S.M.; Silva-Filho, E.V.; Pereira, R.G.E.; Miekeley, N. Accumulation of Metals in Macrophytes From Water Reservoirs of a Power Supply Plant, Rio De Janeiro State, Brazil. Water Air Soil Pollut. 2007, 178, 89–102. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Badr, N.E.; El-Khatib, A.; Abo-El-Kassem, A. Heavy Metal Biomonitoring and Phytoremediation Potentialities of Aquatic Macrophytes in River Nile. Environ. Monit. Assess. 2012, 184, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Curtis, P.S. The Meta-Analysis of Response Ratios in Experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chen, F.; Hsu, M.J. Threat of Heavy Metal Pollution in Halophytic and Mangrove Plants of Tamil Nadu, India. Environ. Pollut. 2008, 155, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.; Olkin, I. Meta Analysis: A Review and a New View. Educ. Res. 1986, 15, 14–16. [Google Scholar] [CrossRef]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. Metawin: Statistical Software for Meta-Analysis with Resampling Tests. Available online: http://psycnet.apa.org/psycinfo/1997-09001-000 (accessed on 12 September 2015).
- Schwarzer, G.; Carpenter, J.; Rücker, G. Empirical Evaluation Suggests Copas Selection Model Preferable to Trim-and-Fill Method for Selection Bias in Meta-Analysis. J. Clin. Epidemiol. 2010, 63, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, H.; Musch, J.; Pietrowsky, R. Publication Bias in Meta-Analyses of the Efficacy of Psychotherapeutic Interventions for Schizophrenia. Schizophr. Res. 2012, 138, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Madejon, P.; Maranon, T.; Murillo, J.M.; Robinson, B. White Poplar (Populus Alba) as a Biomonitor of Trace Elements in Contaminated Riparian Forests. Environ. Pollut. 2004, 132, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Gallé, A.; Florez-Sarasa, I.; Perdomo, J.A.; Galmés, J.; Ribas-Carbó, M.; Flexas, J. Assessment of the Role of Silicon in the Cu-Tolerance of the C4 Grass Spartina Densiflora. J. Plant Physiol. 2015, 178, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jiang, X.; Lv, Y.; Zhou, J.; Yuan, L.; Jia, Y.; Wang, Y. Long-Term Effect of Cu (Ii) On the Phosphorous Removal Performance in Enhanced Biological Phosphorous Removal Systems. Chem. Eng. J. 2015, 281, 164–173. [Google Scholar] [CrossRef]
- Rosenzweig, A.C. Metallochaperones: Bind and Deliver. Chem. Biol. 2002, 9, 673–677. [Google Scholar] [CrossRef]
- O’Halloran, T.V.; Culotta, V.C. Metallochaperones, an Intracellular Shuttle Service for Metal Ions. J. Biol. Chem. 2000, 275, 25057–25060. [Google Scholar] [CrossRef] [PubMed]
- Samecka-Cymerman, A.; Kempers, A.J. Concentrations of Heavy Metals and Plant Nutrients in Water, Sediments and Aquatic Macrophytes of Anthropogenic Lakes (Former Open Cut Brown Coal Mines) Differing in Stage of Acidification. Sci. Total Environ. 2001, 281, 87–98. [Google Scholar] [CrossRef]
- Lesage, E.; Rousseau, D.P.; Meers, E.; Tack, F.M.; De Pauw, N. Accumulation of Metals in a Horizontal Subsurface Flow Constructed Wetland Treating Domestic Wastewater in Flanders, Belgium. Sci. Total Environ. 2007, 380, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Vehla, J.; Kr Pfelová, L.; Chrastny, V. Trace Metals in Phragmites australis and Phalaris Arundinacea Growing in Constructed and Natural Wetlands. Sci. Total Environ. 2007, 380, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mcgrath, S.P.; Shen, Z.G.; Zhao, F.J. Heavy Metal Uptake and Chemical Changes in the Rhizosphere of Thlaspi Caerulescens and Thlaspi Ochroleucum Grown in Contaminated Soils. Plant Soil 1997, 188, 153–159. [Google Scholar] [CrossRef]
- Saraswat, S.; Rai, J.P.N. Heavy Metal Adsorption from Aqueous Solution Using Eichhornia crassipes Dead Biomass. Int. J. Miner Process 2010, 94, 203–206. [Google Scholar] [CrossRef]
- Ederli, L.; Reale, L.; Ferranti, F.; Pasqualini, S. Responses Induced by High Concentration of Cadmium in Phragmites australis Roots. Physiol. Plantarum. 2004, 121, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, K.S.; Kang, D.; Yoon, H.; Sung, K. Effects of Humic Acid On Heavy Metal Uptake by Herbaceous Plants in Soils Simultaneously Contaminated by Petroleum Hydrocarbons. Environ. Earth Sci. 2013, 68, 2375–2384. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Fukegawa, D.; Hayakawa, S.; Mine, A.; Nakamura, M.; Minagi, S.; Osaka, A.; Suzuki, K.; et al. Nano-Controlled Molecular Interaction at Adhesive Interfaces for Hard Tissue Reconstruction. Acta Biomater. 2010, 6, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P. The Haber-Weiss Reaction and Mechanisms of Toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on Cadmium Toxicity in Plants: A Review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis Thaliana to Cd Or Cu Excess Leads to Oxidative Stress Mediated Alterations in Mapkinase Transcript Levels. Environ. Exp. Bot 2012, 83, 53–61. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to Cope with Arsenic or Cadmium Excess in Plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, S.; Norvell, W.A.; McBride, M.; Hendershot, W. Speciation and Complexation of Cadmium in Extracted Soil Solutions. Environ. Sci. Technol. 2000, 34, 291–296. [Google Scholar] [CrossRef]
- Clemens, S. Toxic Metal Accumulation, Responses to Exposure and Mechanisms of Tolerance in Plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Rai, U.N.; Sinha, S.; Tripathi, R.D.; Chandra, P. Wastewater Treatability Potential of some Aquatic Macrophytes: Removal of Heavy Metals. Ecol. Eng. 1995, 5, 5–12. [Google Scholar] [CrossRef]
- Sahu, R.K.; Naraian, R.; Chandra, V. Accumulation of Metals in Naturally Grown Weeds (Aquatic Macrophytes) Grown On an Industrial Effluent Channel. Clean Soil Air Water 2007, 35, 261–265. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal Uptake, Transport and Release by Wetland Plants: Implications for Phytoremediation and Restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.; Barceló, J.; Bernal, M.P.; Navari-Izzo, F.; Poschenrieder, C.; Shilev, S.; Clemente, R.; Monterroso, C. Trace Element Behaviour at the Root–Soil Interface: Implications in Phytoremediation. Environ. Exp. Bot 2009, 67, 243–259. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, C.R.; Tu, C.; Shen, Z.G. Chemical Methods and Phytoremediation of Soil Contaminated with Heavy Metals. Chemosphere 2000, 41, 229–234. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The Influence of Ph and Organic Matter Content in Paddy Soil on Heavy Metal Availability and their Uptake by Rice Plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Human-Caused Environmental Change: Impacts on Plant Diversity and Evolution. Proc. Natl. Acad. Sci. USA 2001, 98, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Albers, P.H.; Camardese, M.B. Effects of Acidification on Metal Accumulation by Aquatic Plants and Invertebrates. 1. Constructed Wetlands. Environ. Toxicol. Chem. 1993, 12, 959–967. [Google Scholar] [CrossRef]
- Yurukova, L.; Kochev, K. Heavy Metal Concentrations in Freshwater Macrophytes from the Aldomirovsko Swamp in the Sofia District, Bulgaria. Bull. Environ. Contam. Toxicol. 1994, 52, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Chapter 15—Phytoextraction: The Use of Plants to Remove Heavy Metals from Soil. Available online: http://www.sciencedirect.com/science/article/pii/B9780128031582000151 (accessed on 22 November 2015).
- Soudek, P.; Petrová, A.; Vaňková, R.; Song, J.; Vaněk, T. Accumulation of Heavy Metals Using Sorghum Sp. Chemosphere 2014, 104, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zaranyika, M.F.; Gurira, L.M.R.C. Cyanide Ion Concentration in the Effluent From Two Gold Mines in Zimbabwe and in a Stream Receiving Effluent From One of the Goldmines. J. Environ. Sci. Health A 1994, 29, 1295–1303. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yu, H.; Luan, Y. Meta-Analysis of the Copper, Zinc, and Cadmium Absorption Capacities of Aquatic Plants in Heavy Metal-Polluted Water. Int. J. Environ. Res. Public Health 2015, 12, 14958-14973. https://doi.org/10.3390/ijerph121214959
Li J, Yu H, Luan Y. Meta-Analysis of the Copper, Zinc, and Cadmium Absorption Capacities of Aquatic Plants in Heavy Metal-Polluted Water. International Journal of Environmental Research and Public Health. 2015; 12(12):14958-14973. https://doi.org/10.3390/ijerph121214959
Chicago/Turabian StyleLi, Jing, Haixin Yu, and Yaning Luan. 2015. "Meta-Analysis of the Copper, Zinc, and Cadmium Absorption Capacities of Aquatic Plants in Heavy Metal-Polluted Water" International Journal of Environmental Research and Public Health 12, no. 12: 14958-14973. https://doi.org/10.3390/ijerph121214959






