Polycyclic Aromatic Hydrocarbons in the Dagang Oilfield (China): Distribution, Sources, and Risk Assessment
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling
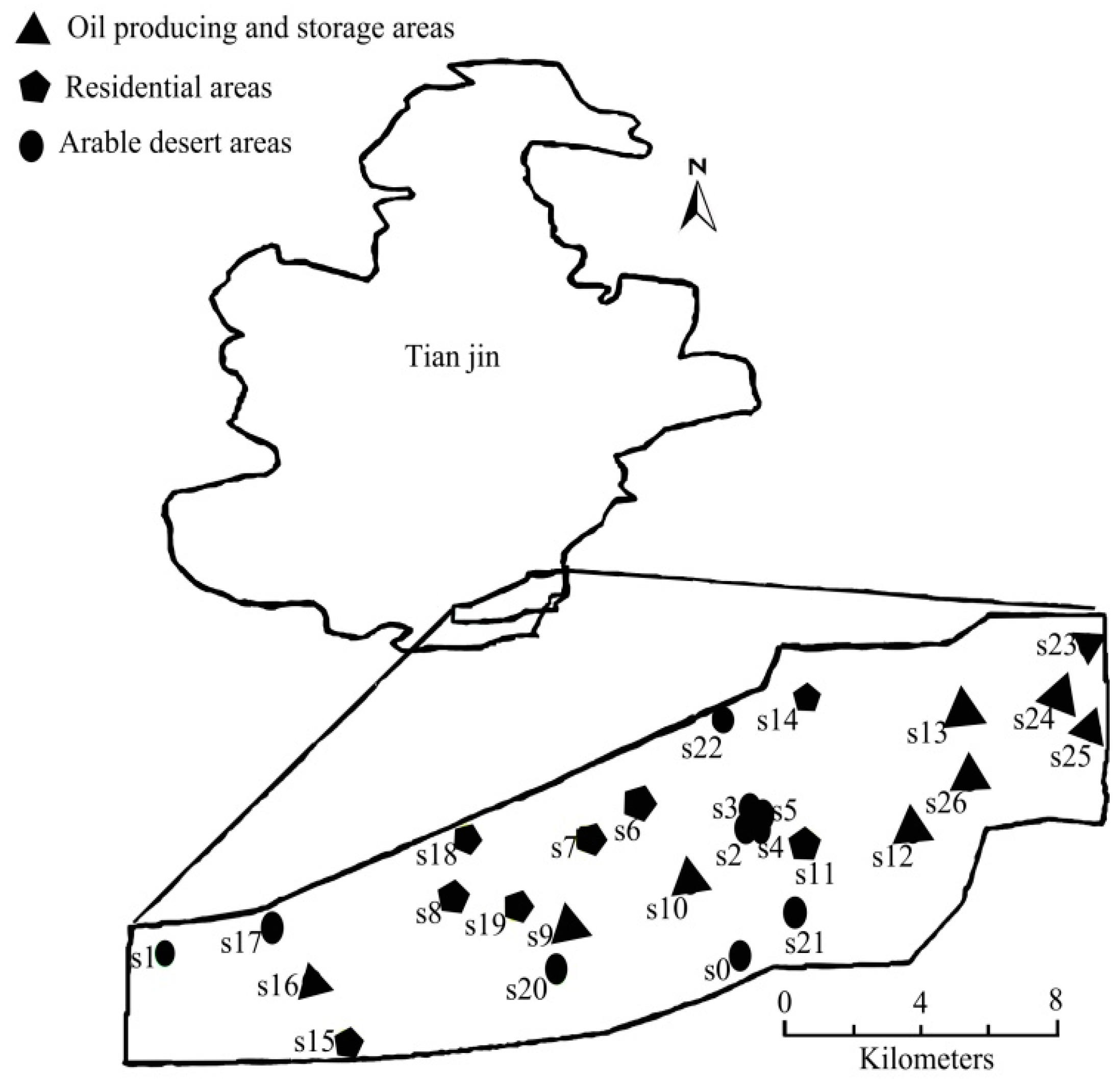
2.2. Reagents and Instruments
2.3. Methodology for Determination of PAHs
2.4. Quality Control
2.5. Statistics
3. Results and Discussion
3.1. PAHs Concentrations in Soil Samples
| PAH | Arable Desert Soil Area (AD) | |||||||||
| S0 | S1 | S2 | S3 | S4 | S5 | S17 | S20 | S21 | S22 | |
| Nap | 283.6 | 2.26 | 476.0 | 37.41 | 505.6 | 478.5 | ND | ND | ND | ND |
| Baa | ND | ND | 7.11 | 6.99 | ND | ND | 0.49 | 0.9 | 0.83 | 0.46 |
| BkF | 4.13 | ND | ND | ND | ND | 4.13 | 3.09 | 3.13 | 4.05 | 2.39 |
| BaP | 4.98 | ND | 5.55 | ND | ND | 5.6 | 5.09 | 5.00 | 5.16 | 4.65 |
| Ind | 15.13 | 15 | ND | ND | ND | 15.1 | 15.11 | 6.13 | 15.82 | 7.13 |
| Ace | ND | ND | ND | ND | ND | ND | ND | ND | 0.21 | ND |
| Flu | 8.65 | 35.27 | 3.39 | 5.59 | 9.19 | 6.53 | 15.02 | 14.53 | 24.88 | 16.58 |
| Phe | 22.82 | 34.22 | ND | 33.88 | 52.36 | 25.03 | 54.27 | 108.70 | 218.7 | 196.2 |
| Ant | 20.26 | 0.21 | ND | 0.19 | 0.21 | 0.22 | ND | ND | ND | ND |
| Fla | 9.43 | 12.23 | 9.46 | 34.72 | 32.21 | 17.03 | 10.37 | 21.85 | 93.24 | 25.42 |
| Pyr | 3.52 | 3.60 | 3.6 | 24.31 | 50.0 | 3.6 | 6.86 | 28.88 | 116.2 | 27.93 |
| Bghip | 0.30 | 0.80 | 0.20 | 0.10 | 0.80 | 1.10 | 7.19 | 7.46 | 13.93 | 8.46 |
| ∑PAHs | 372.8 | 103.6 | 505.3 | 143.2 | 650.3 | 556.8 | 117.5 | 196.6 | 492.9 | 289.2 |
| ∑BPAHs | 307.8 | 17.3 | 488.7 | 44.4 | 505.6 | 503.3 | 23.8 | 15.2 | 25.9 | 14.6 |
| PAHs | Around oil well areas (OW) | |||||||||
| S9 | S10 | S12 | S13 | S16 | S23 | S24 | S25 | S26 | - | |
| Nap | 489.8 | 689.8 | 738.5 | 471.3 | ND | ND | ND | ND | 20.85 | - |
| Chr | ND | ND | ND | ND | ND | ND | ND | 199.6 | 203.7 | - |
| Baa | ND | ND | 7.12 | 7.1 | 0.68 | 0.52 | 0.47 | 73.42 | 0.89 | - |
| BkF | ND | 4.15 | ND | 4.14 | 2.02 | 3.13 | 3.6 | 92.78 | 4.05 | - |
| BaP | ND | 0 | 5.58 | 6.03 | 5.47 | 4.54 | 5.62 | 23.85 | 23.85 | - |
| Ind | ND | 16.0 | ND | ND | 7.13 | 5.13 | 6.13 | 15.13 | 15.82 | - |
| Ace | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Flu | 9.97 | 3.39 | 7.44 | 5.21 | 15.78 | 11.67 | 13.82 | 18.22 | 129.1 | - |
| Phe | 52.33 | ND | 23.13 | 26.10 | 53.10 | 140.3 | 90.59 | 268.4 | 512.5 | - |
| Ant | 0.23 | ND | 0.25 | 0.27 | ND | ND | ND | ND | ND | - |
| Fla | 9.43 | 9.41 | 9.43 | 18.55 | 18.77 | 19.88 | 16.05 | 157.05 | 48.11 | - |
| Pyr | 24.31 | 3.59 | 17.63 | 55.97 | 19.38 | 25.11 | 198.7 | 175.6 | 46.80 | - |
| Bghip | 7.70 | 9.20 | 8.30 | 6.90 | 6.93 | 6.77 | 5.66 | 189.4 | 17.4 | - |
| ∑PAHs | 593.8 | 735.6 | 817.4 | 601.6 | 129.3 | 217.0 | 340.6 | 1214 | 1029 | - |
| ∑BPAHs | 489.8 | 710.1 | 751.2 | 488.6 | 15.30 | 13.32 | 15.82 | 404.7 | 269.2 | - |
| PAHs | Resident (RD) | |||||||||
| S6 | S7 | S8 | S11 | S14 | S15 | S18 | S19 | - | - | |
| Nap | 517.3 | 460.5 | 571.4 | 602.8 | 548.7 | 2.75 | 0.37 | 26.73 | - | - |
| Chr | ND | ND | 52.96 | 288.9 | ND | ND | ND | 2966.1 | - | - |
| Baa | ND | 7.01 | 47.70 | 397.8 | 7.13 | 0.86 | 0.91 | 0.87 | - | - |
| BkF | 4.10 | 3.99 | 4.13 | 495.2 | 4.10 | 4.13 | 4.97 | 3.97 | - | - |
| BaP | 5.70 | 5.71 | 0.00 | 427.1 | ND | 5.65 | 4.95 | 1384.1 | - | - |
| Ind | 15.13 | ND | 57.70 | 410.9 | ND | 1579.8 | 6.26 | 15.40 | - | - |
| Ace | ND | ND | ND | 15.02 | ND | ND | ND | 2.86 | - | - |
| Flu | 3.39 | 7.01 | 11.50 | 28.36 | 11.07 | 45.01 | 32.46 | 168.5 | - | - |
| Phe | 28.03 | 15.12 | 89.62 | 268.4 | 43.67 | 269.8 | 164.9 | 374.7 | - | - |
| Ant | 0.25 | 0.20 | 0.22 | 39.08 | 0.26 | ND | ND | ND | - | - |
| Fla | 39.45 | 55.04 | 89.02 | 411.9 | 6.28 | 54.62 | 71.17 | 806.5 | - | - |
| Pyr | 3.59 | 12.80 | 87.65 | 379.9 | 34.03 | 41.60 | 31.52 | 105.91 | - | - |
| Bghip | 1.20 | 5.20 | 8.60 | 17.05 | 5.89 | 17.46 | 17.61 | 16.19 | - | - |
| ∑PAHs | 618.1 | 572.5 | 1021 | 3783 | 661.1 | 2022 | 335.1 | 5872 | - | - |
| ∑BPAHs | 542.2 | 477.2 | 733.9 | 2623 | 559.9 | 1593 | 17.46 | 4397 | - | - |
3.2. Composition and Profiles of PAHs in Individual Sampling Sites


3.3. Analysis of Potential Contamination Sources
3.3.1. Isomer Ratios

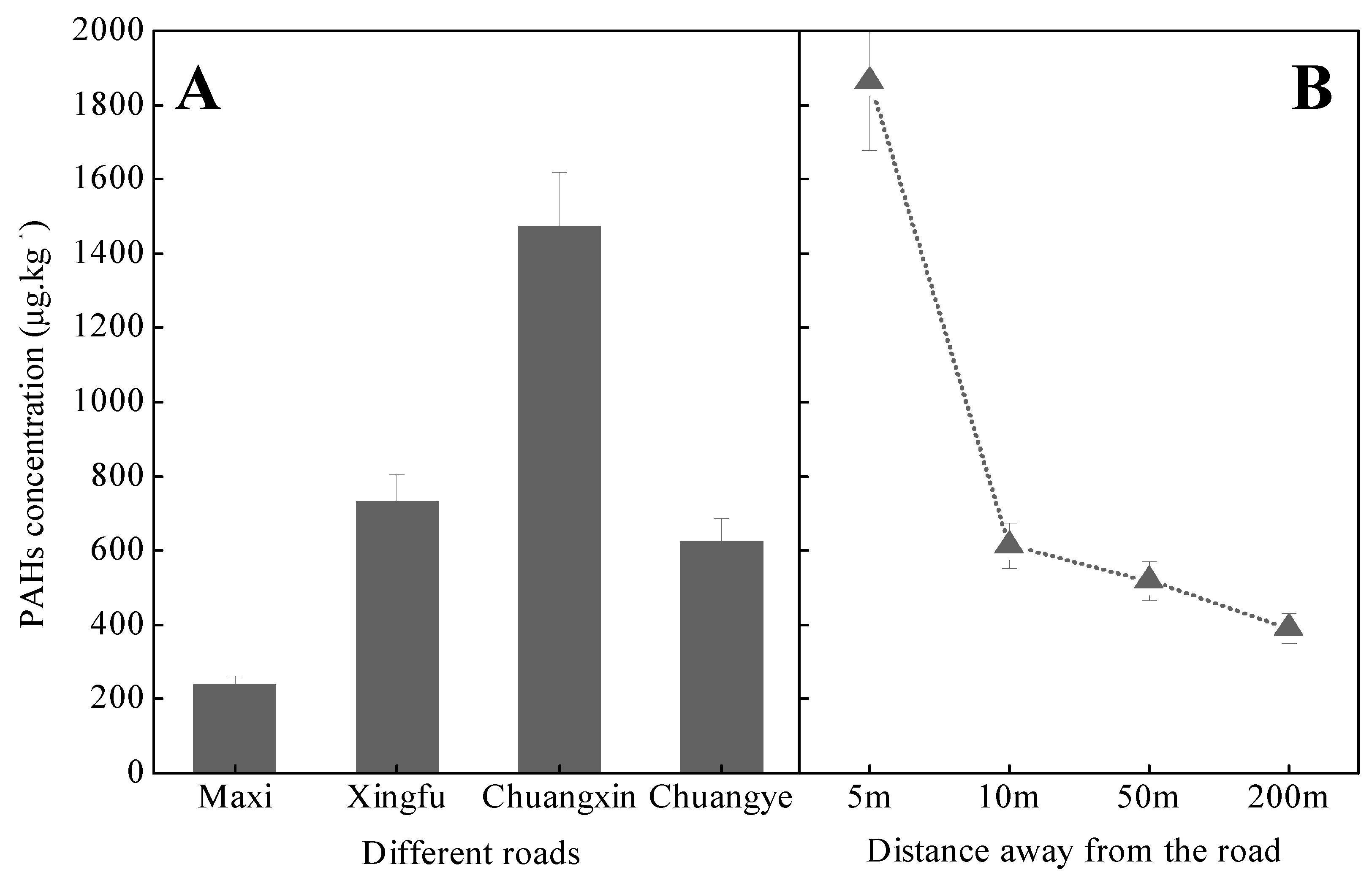
3.3.2. Relationship to Anthropogenic Activities

3.4. Assessment of Soil Toxicity

| Compound | USEPA | Nisbet And LaGoy | This Study | Carcinogenicity Classification |
|---|---|---|---|---|
| Nap | 0 | 0.001 | 0.001 | B a |
| Ace | 0 | 0.001 | 0.001 | |
| AcP | 0 | 0.001 | 0.001 | |
| Flu | 0 | 0.001 | 0.001 | |
| Phe | 0 | 0.001 | 0.001 | |
| Ant | 0 | 0.01 | 0.01 | |
| Fla | 0 | 0.001 | 0.001 | |
| Pyr | 0 | 0.001 | 0.001 | |
| Baa | 1 | 0.1 | 1 | B |
| Chr | 1 | 0.01 | 1 | B |
| Bbf | 1 | 0.1 | 0.1 | B |
| BkF | 1 | 0.1 | 1 | B |
| BaP | 1 | 1 | 1 | B |
| DahA | 1 | 1 | 1 | B |
| BghiP | 0 | 0.01 | 0.01 | |
| Inp | 1 | 1 | 1 | B |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilcke, W. Polycyclic aromatic hydrocarbons (PAHs) in soil-A review. J. Plant. Nutr. Soil Sci. 2000, 163, 229–248. [Google Scholar] [CrossRef]
- Wild, S.R.; Jones, K.C. Polynuclear aromatic hydrocarbons in the United Kingdom environment: A preliminary source inventory and budget. Environ. Pollut. 1995, 88, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Rieth, S.H.; Cogliano, V.J.; Foureman, G.L.; Hertzberg, R.; Hofmann, E.L.; Murphy, D.L.; Nesnow, S.; Schoeny, R.S. Health assessment of polycyclic aromatichydrocarbon mixtures: Current practices and future directions. Polycycl. Aromat. Compd. 2002, 22, 811–821. [Google Scholar] [CrossRef]
- National Center for Environmental Assessment Office of Research and DevelopmentUnited States Environmental Protection AgencyExposure Factors Handbook, 1997 Final Report. Available online: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=12464 (accessed on 20 May 2015).
- Blasco, M.; Domeño, C.; Nerín, C. Use of lichens as pollution bio-monitors in remote areas: Comparison of PAHs extracted from lichens and atmospheric particles sampled in and around the Somport tunnel (Pyrenees). Environ. Sci. Technol. 2006, 40, 6384–6391. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Baran, S. Polycyclic aromatic hydrocarbons content in shoots and leaves of willow (salixviminalis) cultivated on the sewage sludge-amended soil. Water Air Soil Pollut. 2005, 168, 91–111. [Google Scholar] [CrossRef]
- Xiao, Y.; Tong, F.; Kuang, Y.; Chen, B. Distribution and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in forest soils from urban to rural Areas in the Pearl River delta of Southern China. Int. J. Environ. Res. Public Health 2014, 11, 2642–2656. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Khillare, P.S.; Shridhar, V.; Ray, S. Pattern, sources and toxic potential of PAHs in the agriculturalsoils of Delhi, India. J. Hazard. Mater. 2009, 163, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A.; Terelak, H. Monitoring of the total content of polycyclic aromatic hydrocarbons (PAHs) in arable soils in Poland. Chemosphere 2008, 73, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Sverdrup, L.E.; Nielsen, T.; Krogh, P.H. Soil ecotoxicity of polycyclic aromatic hydrocarbons in relation to soil sorption, lipophilicity, and water solubility. Environ. Sci. Technol. 2002, 36, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, R.; Niu, X.; Zhou, Q. Enhancement of soil petroleum remediation by using a combination of ryegrass (Loliumperenne) and different microorganisms. Soil Tillage Res. 2010, 110, 87–93. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Method 3540C, Soxhlet Extraction. 1996. Available online: http://en.wikipedia.Org/wiki/United_States_Environmental_Protection_ Agency (accessed on 20 May 2015).
- Maliszewska-Kordybach, B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl. Geochem. 1996, 11, 121–127. [Google Scholar] [CrossRef]
- Kuang, S.; Wu, Z.; Zhao, L. Accumulation and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soils around oil sludge in Zhongyuan Oilfield, China. Environ. Earth Sci. 2011, 64, 1353–1362. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Breivik, K. Model-based evaluation of the use of polycyclic aromatic hydrocarbons molecular diagnostic ratios as a source identification tool. Environ. Pollut. 2014, 184, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, A.; Terzi, E.; Cai, Q.-Y. On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere 2007, 69, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-F.; Chen, C.-W. Determination of polycyclic aromatic hydrocarbons in industrial harbor sediments by GC-MS. Int. J. Environ. Res. Public Health 2012, 9, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Chen, C.-F. Distribution, origin and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor, Taiwan. Mar. Pollut. Bull. 2011, 63, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xia, X.; Yang, Z.; Wang, R.; Voulvoulis, N. Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environ. Pollut. 2006, 144, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.P.; Takada, H.; Tsutsumi, S.; Ohno, K.; Yamada, J.; Kouno, E.; Kumata, H. Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs. Environ. Sci. Technol. 2002, 36, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Tay, C.K.; Biney, C.A. Levels and sources of polycyclic aromatic hydrocarbons (PAHs) in selected irrigated urban agricultural soils in Accra, Ghana. Environ. Earth Sci. 2013, 68, 1773–1782. [Google Scholar] [CrossRef]
- Sicre, M.A.; Malty, J.C.; Saliot, A.; Aparicio, X.; Grimalt, J.O.; Albaiges, J. Aliphatic and aromatic hydrocarbons in the Medi-terranean aerosol. Int. J. Environ. Anal. Chem. 1987, 29, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Sokhi, R.; van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Hao, R.; Wan, H.-F.; Song, Y.-T.; Jiang, H.; Peng, S.-L. Polycyclic aromatic hydrocarbons in agricultural soils of the southern subtropics, China. Pedosphere 2007, 17, 673–680. [Google Scholar] [CrossRef]
- Peng, C.; Chen, W.; Liao, X.; Wang, M.; Ouyang, Z.; Jiao, W.; Bai, Y. Polycyclic aromatic hydrocarbons in urban soils of Beijing: Status, sources, distribution and potential risk. Environ. Pollut. 2011, 159, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-F.; Wang, X.-T.; Wang, F.; Jia, Y.; Wu, M.-H.; Sheng, G.-Y.; Fu, J.-M. Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere 2009, 75, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Cachada, A.; Pato, P.; Rocha-Santos, T.; da Silva, E.F.; Duarte, A.C. Levels, sources and potential human health risks of organic pollutants in urban soils. Sci. Total Environ. 2012, 430, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Hu, R.; Cheung, K.C.; Wong, M.H. Pollutants in Hongkong soils: Polycyclic aromatic hydrocarbons. Chemosphere 2007, 67, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-L.; Li, Y.-F.; Sun, D.-Z.; Qi, H. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in top soils of Harbin, China. Arch. Environ. Contam. Toxicol. 2009, 57, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environ. Pollut. 2004, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). Guidelines for ecological risk assessment. Fed. Regist. 1998, 63, 846–924.
- Nisbet, I.C.T.; LaGoy, P. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmocol. 1992, 16, 290–300. [Google Scholar]
- Environment Canada, Toxicity Testing of National Contaminated Site Remediation Program Priority Substances for Development of Soil Quality Criteria for Contaminated Sites; Technical Report for Environmental Conservation Service: Ottawa, Canada, 1995.
- Liu, S.; Xia, X.; Yang, L.; Shen, M.; Liu, R. Polycyclic aromatic hydrocarbons in urban soils of different land uses in Beijing, China: Distribution, sources and their correlation with the city’s urbanization history. J. Hazard. Mater. 2010, 177, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, H.; Rui, X.; Wu, S.; Bai, Z.; Zhuang, X.; Huang, Z. Polycyclic Aromatic Hydrocarbons in the Dagang Oilfield (China): Distribution, Sources, and Risk Assessment. Int. J. Environ. Res. Public Health 2015, 12, 5775-5791. https://doi.org/10.3390/ijerph120605775
Jiao H, Rui X, Wu S, Bai Z, Zhuang X, Huang Z. Polycyclic Aromatic Hydrocarbons in the Dagang Oilfield (China): Distribution, Sources, and Risk Assessment. International Journal of Environmental Research and Public Health. 2015; 12(6):5775-5791. https://doi.org/10.3390/ijerph120605775
Chicago/Turabian StyleJiao, Haihua, Xiaoping Rui, Shanghua Wu, Zhihui Bai, Xuliang Zhuang, and Zhanbin Huang. 2015. "Polycyclic Aromatic Hydrocarbons in the Dagang Oilfield (China): Distribution, Sources, and Risk Assessment" International Journal of Environmental Research and Public Health 12, no. 6: 5775-5791. https://doi.org/10.3390/ijerph120605775







