Potential Occupational Exposures and Health Risks Associated with Biomass-Based Power Generation
Abstract
:1. Introduction
2. Summary of Available Technologies and Fuel Types
| Direct Fired Technology | Common Fuel Types | Biomass Feed Size (cm) | Moisture Content (%) | Generation Capacity (MW) |
|---|---|---|---|---|
| Pile burners | Wood or agricultural residues (excl. wood flour) | Limited by grate size and feed opening | <65 | 4 to 110 |
| - with underfire stoker | Sawdust, select bark (“non-stringy”), shavings, chips, “hog” fuel | 0.6–5 | 10–30 | 4 to 110 |
| Stoker grate boilers | Sawdust, select bark (“non-stringy”), shavings, end cuts, chips, “hog” fuel, sander dust | 0.6–5 | 10–50 | 4 to 300 |
| Suspension boilers | ||||
| - Cyclonic | Sawdust, select bark (“non-stringy”), shavings, wood flour, sander dust | <0.6 | <15 | <30 |
| - Air spreader-stoker | Wood flour, sander dust, processed sawdust, shavings | 0.1–0.15 | <20 | 1.5 to 30 |
| Fluidized-bed combustor | Low alkali fuels: wood residues or peat | <5 | <60 | Up to 300 |
| - with underfire stoker | Sawdust, select bark (“non-stringy”), shavings, chips, “hog” fuel | 0.6–5 | 10–30 | 4 to 110 |
| - with underfire stoker | Sawdust, select bark (“non-stringy”), shavings, chips, “hog” fuel |
| Air Pollution Control or Environmental Target | Emission Control Options | |
|---|---|---|
| Stoker Boiler | Fluidized Bed Boiler | |
| Typical post-combustion air pollution control | PM—Cyclones, ESP, FF NOx—SNCR, SCR (only applicable for low alkali fuels) CO—oxidation catalysis SOx/HCl—IDSIS, SDA, DS (with FF), FGDw | PM—ESP and FF NOx—SNCR, SCR (only applicable for low alkali fuels) CO—generally absent SOx/HCl—In furnace injection, IDSIS, SDA, DS, FGDd (with FF) |
| Low sulfur oxide (SOx) combustion | Not possible (in furnace) | Some reduction possible through limestone addition to bed material |
| Low NOx combustion | Air staging | Generally low inherent NOx (due to lower temperature), air staging, flue gas recirculation |
| Low CO formation | Difficult (lower combustion efficiency) | Generally low due to higher combustion efficiency |
3. Potential Occupational Exposures
3.1. Overview of Exposure Sources and Routes
3.2. Substances of Significance to Health
3.2.1. Pre-Combustion Exposures
3.2.2. Combustion-Related Exposures
3.2.3. Post-Combustion Related Exposures
| Job Type | Tasks | Potential Exposures |
|---|---|---|
| Trucker | Transport of biomass to site (road/rail) Loading and discharge of material Transport of ash | Biomass dust and bioaerosols generated during biomass loading and discharge Ash dust generated during loading and discharge Diesel exhaust from vehicles |
| Fuel Handling Plant operative | Transport of biomass through the site Storage of biomass Fuel preparation (milling etc.) | Biomass dust and bioaerosols generated during biomass handling and milling Off-gases from storage Direct contact with moldy biomass |
| Cleaner | Removal of dust deposits from plant | Generation of airborne biomass dust, bioaerosols and ash through disturbance of deposits Potential for direct contact with moldy biomass |
| Maintenance engineer | Maintenance of plant equipment during normal operation | Generation of airborne biomass dust, bioaerosols and ash through disturbance of deposits Potential for exposure to combustion gases |
| Outage contractor | Repair of plant items during shutdown periods (particularly within the boiler) | Generation of airborne biomass dust, bioaerosols and ash through disturbance of deposits Direct contact with ash deposits within the boiler (often confined spaces) |
| Ash handling plant operative | Removal of ash from the boiler Transport to storage | Direct contact with ash |
| Other plant personnel | Various | Fugitive dusts from fuel and ash handling plants Combustion gases |
| SSH Class | COI | Source | Industry | Reference(s) |
|---|---|---|---|---|
| Particulate Matter | Wood dust | Raw or processed material Straw, wood chips, pellets | Forestry Wood pellet production Biomass generation Biomass laboratory | [25,37,38,39,40] |
| Bioaerosols | Microbial (Fungi/Bacteria) | Component of PM Wood chips or pellets | Biomass power generation Fuel processing and handling | [23,40,41,42,43] |
| Endotoxin | Component of PM Straw, grain, hay, organic waste | Biomass power generation | [21] | |
| Volatile Organics (VOCs) | Aldehydes Total VOCs | Off gassing from sawdust Auto-oxidation of unsaturated fatty acids | Wood pellet production | [5,26] |
| Organics | Monoterpenes Resin acids | Components of PM, off gassing from sawdust | Wood pellet production Forestry, milling | [5,26,44] |
| Inorganic Gases | Carbon monoxide | Off gassing from raw materials | Wood pellet production, transport, storage | [28,29,30] |
| SSH Class | SSH | Source | Refs | Health Effects Associated with Exposure Route | Refs | |
|---|---|---|---|---|---|---|
| Inhalation | Dermal/Eye | |||||
| Inorganic Gases | Carbon monoxide | Combustion | [45] | CNS; Miscarriage; Carboxylhemoglobinemia | [45,46] | |
| Nitrogen oxides | Combustion | [45] | URT and LRT | Irritation (Skin and Eye) | [45,48] | |
| Sulfur oxides | Combustion | [46] | Pulmonary function; LRT | [45,49] | ||
| Acid aerosols (e.g., H2SO4) | Combustion | [47] | Pulmonary function | Irritation (Skin and Eye) | [45,49] | |
| Hydrocarbons | 1,3-Butadiene | Combustion | [45] | CNS; Stomach, Respiratory and Hematolymphopoietic Cancers | [45,50] | |
| n-Hexane | Combustion | [45] | CNS; Peripheral Neuropathy | Irritation (Eye) | [45] | |
| PAHs a | Combustion, Ash | [45,48,49] | Lung Cancer | Skin Cancer * | [51] | |
| Benzene | Combustion | [45] | Leukemia; Anemia; CNS | [45,52] | ||
| Styrene | Combustion | [45] | CNS | [45] | ||
| Oxygenated organics | Acrolein | Combustion | [45] | URT; Pulmonary edema; Pulmonary emphysema | Irritation (Skin and Eye) | [45] |
| Formaldehyde | Combustion | [45] | URT; Nose Cancer * | Irritation (Skin and Eye) | [45,53] | |
| Methanol | Combustion | [45] | CNS; URT | Eye Damage | [45,54] | |
| Acetic acid | Combustion | [45] | URT; Pulmonary function | Irritation (Eye) | [45] | |
| Catechol | Combustion | [45] | URT | Dermatitis; Irritation (Eye) | [45] | |
| Cresol (methylphenols) | Combustion | [45] | URT; Kidney; Liver | Skin Damage | [45,55] | |
| Hydroquinone | Combustion | [45] | CNS | Irritation (Eye) | [45,56] | |
| Fluorenone | Combustion | [45] | URT | Irritation (Eye) | [57] | |
| Anthraquinone | Combustion | [45] | Respiratory | Irritation (Skin and Eye) | [58] | |
| Chlorinated organics b | Methylene chloride | Combustion | [45] | CNS; Peripheral Neuropathy; Liver and Lung Cancer * | Irritation (Skin and Eye) | [59,60] |
| Methyl chloride | Combustion | [45] | CNS; Liver; Kidney; CNS *; Testicular *; Teratogenic * | [45,61] | ||
| Dioxins/furans | Combustion | [45,48] | URT; Chloracne; Liver; Glucose metabolism | Chloracne | [62,63] | |
| Particulate matter (PM) | PM10 | Combustion/Condensation | [45] | Pulmonary function; URT | Irritation (Eye) | [64] |
| PM2.5 | Combustion/Condensation | [45] | Pulmonary function; URT | Irritation (Eye) | [22] | |
| Inorganics | Aluminum (Al) c | Combustion | [45] | Pneumoconiosis; LRT | [45,66] | |
| Arsenic (As) e | Ash | [48,49] | URT and LRT; Lung Cancer | [45,67] | ||
| Beryllium (Be) d | Ash | [48] | Beryllium disease; | Irritation (Skin) | [45,68,69] | |
| Cobalt (Co) d,e | Ash | [48] | Pulmonary function; Myocardial effects | [45,70] | ||
| Magnesium (Mg) d | Combustion | [45] | URT; Pulmonary function; Metal fume fever | Irritation (Eye) | [71] | |
| Iron (Fe) d | Combustion | [45,49] | Pneumoconiosis; URT | Irritation (Skin and Eye) | [45,72] | |
| Manganese (Mn) f | Combustion | [45] | Neurobehavioral | [73,74] | ||
| Zinc (Zn) h | Combustion | [45,49] | Metal fume fever; LRT and URT | Irritation (Skin and Eye) | [45,75,76] | |
| Nickel (Ni) d | Combustion, Ash | [45,48,49] | Pneumoconiosis; Nasal and Lung Cancer | Dermatitis | [45,77,78] | |
| Copper (Cu) d | Combustion | [45,49] | URT; Metal fume fever | Irritation (Eye) | [45,79] | |
| Lead (Pb) f,g,h,i | Combustion | [45,49] | CNS and PNS; Hematologic; Nephropathy | [45,80] | ||
| Mercury (Hg) d,f | Ash | [48] | CNS and PNS; Kidney | [45,81] | ||
| Chromium (Cr) d | Combustion, Ash | [45,48,49] | Pulmonary function; Lung Cancer | Irritation (Skin) | [45,82] | |
| Cadmium (Cd) d,i | Combustion | [45] | Pulmonary function; Kidney | [45,83,84] | ||
| Quartz | Ash | [48] | Pulmonary fibrosis; Chronic silicosis; Lung cancer * | [45,85] | ||
| SSH | Madsen et al. [18] N = 1 Concentration (ppm) | Cohn et al. [23] N = 3 Concentration Range (ppm) |
|---|---|---|
| K | 303,154 | − |
| Ca | 53,061 | − |
| Na | 44,266 | − |
| Al | 6789 | − |
| Mg | 5892 | − |
| Fe | 16,434 | 8100–28,000 |
| Mn | 361 | − |
| P | 1890 | − |
| Zn | 1770 | 1050–15,700 |
| Ni | 568 | 30–125 |
| Cu | 530 | 300–525 |
| Pb | 127 | 115–150 |
| Cr | 38 | 20–50 |
| Cd | 5 | − |
| Li | − | 4.8–15 |
| As | − | 5–15 |
| PAH | − | 145–880 |
4. Potential Occupational Risks
4.1. Pre-Combustion Risks
4.1.1. Bioaerosols
4.1.2. Wood Dust
| Wood Name | Classification | Reported Health Effects |
|---|---|---|
| Abura/bahia | Hardwood | vomiting |
| Afrormosia | Hardwood | skin irritation, splinters go septic, nervous system effects |
| Afzelia/doussie | Hardwood | dermatitis, sneezing |
| Agba/tola | Hardwood | skin irritation |
| Alder | Hardwood | dermatitis, rhinitis, bronchial effects |
| Andiroba/crabwood | Hardwood | sneezing, eye irritation |
| Ash | Hardwood | decrease in lung function |
| Avodire | Hardwood | dermatitis, nose bleeds |
| Ayan/movingui | Hardwood | dermatitis |
| Basralocus/angelique | Hardwood | general unspecific effects |
| Beech | Hardwood | dermatitis, decrease in lung function, eye irritation (possibly from bark lichens) |
| Birch | Hardwood | dermatitis on sawing lumber |
| Bubinga | Hardwood | dermatitis, skin lesions possible |
| Cedar of Lebanon | Softwood | respiratory disorders, rhinitis |
| Cedar (Cent/S American) | Hardwood | allergic contact dermatitis |
| Cedar (Western Red) | Softwood | asthma, rhinitis, dermatitis, mucous membrane irritation, central nervous system effects |
| Chestnut (sweet) | Hardwood | dermatitis (possibly from bark lichens) |
| Douglas fir | Softwood | dermatitis, splinters go septic, rhinitis, bronchial effects |
| Ebony | Hardwood | mucous membrane irritation, dermatitis, possibly a skin sensitizer |
| Freijo/cordia | Hardwood | possibly a skin sensitizer |
| Gaboon/okoume | Hardwood | asthma, cough, eye irritation, dermal effects (hands, eyelids) |
| Gedu nohor/edinam | Hardwood | dermatitis (rare) |
| Greenheart | Hardwood | splinters go septic, cardiac and intestinal disorders, severe throat irritation |
| Guarea | Hardwood | skin and mucous membrane irritation |
| Gum (southern blue) | Hardwood | dermatitis |
| Hemlock (western) | Softwood | bronchial effects, rhinitis |
| Idigbo | Hardwood | possible irritant |
| Iroko | Hardwood | asthma, dermatitis, nettle rash |
| Larch | Softwood | nettle rash, dermatitis (possibly from bark lichens) |
| Limba | Hardwood | splinters go septic, nettle rash, nose and gum bleeding, decrease in lung function |
| Mahogany | Hardwood | dermatitis, respiratory disorders, mucous membrane irritation |
| Makore | Softwood | dermatitis, mucous membrane and respiratory tract irritation, central nervous system and blood effects |
| Mansonia | Hardwood | splinters go septic, skin sensitization, irritation, respiratory disorders, nose bleeds, headache, cardiac disorders |
| Maple | Hardwood | decrease in lung function |
| Meranti/lauan (various) | Softwood | skin irritation |
| Oak (various) | Hardwood | asthma, sneezing, eye irritation |
| Obeche | Softwood | skin and respiratory tract irritation, nettle rash, dermatitis (handling articles), feverish, sneezing, wheezing |
| Opepe | Hardwood | dermatitis, mucous membrane irritation, central nervous system effects (e.g., giddiness, visual effects), nose bleeds and blood spitting |
| Padauk | Hardwood | species-dependent: itching, eye irritation, vomiting, swelling (e.g., eyelids) |
| Peroba | Hardwood | skin and mucous membrane irritation, systemic effects (e.g., headache, nausea, stomach cramp, weakness), blisters |
| Pine (many species) | Softwood | skin irritation (may cause photosensitization) decrease in lung function |
| Poplar | Hardwood | sneezing, eye irritation, may cause blisters |
| Ramin | Hardwood | dermatitis (possibly from bark) |
| Rosewood (many species) | Hardwood | dermatitis, respiratory disorders. Effects may arise from handling wood |
| Sapele | Hardwood | skin irritation |
| Spruce (several species) | Softwood | respiratory disorders, possible photosensitization |
| Teak | Hardwood | dermatitis (potent, even after seasoning), nettle rash, respiratory disorders |
| Utile | Hardwood | skin irritation |
| Walnut (not African) | Hardwood | sneezing, rhinitis, dermatitis from nut shells and roots |
| Wenge | Hardwood | splinters go septic, dermatitis, central nervous system effects (e.g., giddiness, drowsiness, visual disturbance), abdominal cramps |
| Whitewood (American) | Hardwood | dermatitis |
| Country/Region | Dust Type | Limits mg/m3 | Additional comments | Health Endpoint/Comments |
|---|---|---|---|---|
| Short-term (15 min) | Long-term (8 h. Time Weighted Average) | |||
| Wood dusts | ||||
| US (OSHA) | Particulate not otherwise regulated (includes wood dust)—inhalable—respirable | 15 5 | Throat, skin, eye irritation, upper respiratory problems | |
| US (NIOSH recommended) | Wood dust | 1 | Pulmonary Function, Carcinogen | |
| European Union (applies to all member countries) | Hardwood (inhalable fraction) | 5 | Carcinogenic, sensitizer | |
| UK | Softwood (inhalable fraction) | 5 | Sensitizer | |
| Australia | Hardwood | 1 | ||
| Australia | Softwood | 5 | ||
| Ontario, Canada | Certain hardwoods such as beech and oak | 1 | ||
| Ontario, Canada | Softwood | 10 | 5 | |
| Sweden | Inhalable non-impregnated wood dust | 2 | Carcinogen | |
| Sweden | Impregnated wood | 0.05 | Applies if levels of impregnating substances (with their own OELs) are unknown | |
| Australia | Softwood | 10 | 5 | Sensitizer |
| Australia | Certain hardwoods such as beech and oak | 1 | Sensitizer | |
| Germany | Respirable wood dust | 2 | Selected species identified as carcinogenic and/or sensitizing | |
| Russia | Wood dust | 6 | Maximum allowable concentration, sensitizer, fibrogenic action | |
| US (OSHA/California) | Wood dust, all soft and hard woods except Western red cedar | 10 | 5 | |
| US (OSHA/California) | Wood dust, Western red cedar | 2.5 | ||
| Other biomass dusts | ||||
| US (OSHA) | Grain dust (oat, wheat, barley) | 10 | ||
| UK | Grain dust (inhalable fraction) | 10 | Sensitizer | |
| Trace metals in biomass ash | ||||
| UK | Cadmium and Cadmium compounds (as Cd) | 0.025 | Carcinogenic (selected compounds) | |
| UK | Cobalt and Cobalt compounds (as Co) | 0.1 | Carcinogenic (selected compounds), sensitizer | |
| UK | Manganese and inorganic manganese compounds (as Mn) | 0.5 | ||
| US (OSHA) | Cadmium dust | 0.5 | 0.2 | |
| US (OSHA) | Cobalt metal, dust, and fume (as Co) | 0.1 | ||
| US (OSHA/California) | Cadmium | 0.005 | ||
| US (California) | Manganese and compounds, as Mn | 0.2 | ||
| US (OSHA/California) | Cobalt metal, dust, and fume (as Co) | 0.02 | ||
4.1.3. Volatile Organic Compounds (VOCs)
4.1.4. Carbon Monoxide (CO)
4.2. Combustion-Associated Risks
4.2.1. Health Effect Studies of Relevance and Uncertainties in the Available Studies
4.2.2. Studies of Occupational Exposures and Potential Health Risks at a Large-Scale Danish Biofuel Plant
4.2.3. Controlled Human Exposure Studies of Small-Scale Biomass Combustion
4.2.4. Epidemiologic Investigations of Uncontrolled Ambient Biomass Smoke
4.2.5. Regulatory Consideration of Biomass Combustion Emissions and Cancer Risk
4.2.6. Conclusions Regarding the Evidence for Biomass Combustion Product Health Risks at Large-Scale Modern Biofuel Facilities
4.3. Post-Combustion Risks
4.3.1. Ash and Inorganic Compounds
4.3.2. Polycyclic Aromatic hydrocarbons (PAHs)
4.3.3. Dioxins/Furans
| Reference | Exposed Population | Combustion Source | Dominating Particle Types | PM2.5 Exposure Levels 1 | Key Statistically Significant Acute Biological Responses 2 | Key Negative Findings 2 |
|---|---|---|---|---|---|---|
| [177,178,179,180] | 13 healthy adults | Small cast iron wood stove Fuel: Standardized mixture (50/50) of hardwood/softwood (birch/spruce), dried for 1 yr (moisture content 15%–18%) Exposure: 4 h | Organic carbon/soot | 240–280 μg/m3 | ↑ Serum amyloid A; ↑ Plasma factor VIII; ↑ Factor VIII/von Willebrand factor ratio; ↑ Urinary excretion of free 8-iso-prostaglandin2α; ↑ Malondialdehyde in breath condensate; ↑ Serum Clara cell protein; ↑ FENO270 and calculated alveolar NO ↓ PBMC levels of DNA strand breaks; ↑ mRNA levels of hOGG1 | “Weak” subjective symptoms; No significant increases in serum C-reactive protein (CRP), fibrinogen, IL-6, or TNF-α levels; No significant changes in RBC, Hb, Hct, leukocytes, or platelets; No significant change FENO50 or NO influx; No significant increase in urinary Clara cell protein No significant changes to FPG sites, hOGG1 activity, or PBMC expression of hNUDT1 or HO-1; No significant changes in urinary excretion of 8-oxodG or 8-oxoGua |
| [181] | 10 healthy adults | Electric element in a woodstove Fuel: Red oak wood Exposure: 2 h | Organic carbon/soot | 485 ± 84 μg/m3 | ↑ Percentage and absolute numbers of neutrophils in blood, BL, and BAL; ↑ IL-1β in blood; ↑ blood LDH c | No significant changes in symptom prevalence or lung function; No significant changes blood or BAL cytokine concentrations (IL-6, IL-8, TNF-α); No significant changes white blood cell counts, blood coagulation (e.g., von Willebrand’s factor, plasminogen activators) or total proteins and albumin; Minimal changes in cardiac endpoints |
| [182] | 26 healthy adults | Standard woodstove Fuel: Dried pine wood with UV aging woodsmoke Exposure: 3 h | Organic carbon/soot | 150–200 μg/m3 | None | No significant changes in vascular function measured by reactive hyperemia-peripheral arterial tonometry (RH-PAT) |
| [183] | 20 healthy adults | Standard woodstove (operated “optimal conditions”) Fuel: Dried beech Exposure: 3 h | Combination of alkali salts, soot, and organic matter | 165–662 μg/m3 | ↑ Self-reported subjective symptoms (significant changes for 5 of 6 indices): “environmental perception” “irritative body perceptions” “psychological/neurological effects” “weak inflammatory” ↑ Self-reported general mucosa irritation | No increase in the index for “lower respiratory effects” |
| [184] | 19 healthy adults | Adjustable wood pellet boiler system (operated under incomplete combustion) Fuel: Moist softwood pellet/sawdust mixture from pine and spruce (18% moisture) | Organic carbon/soot | 224 ± 22 μg/m3 | ↑ Glutathione in BAL; ↑ Upper airway symptoms (nose and throat irritation) | No significant changes in lung function (VC, FVC, FEV1) or exhaled NO (FENO); No significant changes peripheral blood counts; No significant changes GSH in BW or endobronchial biopsy tissue; No significant changes in lung inflammatory parameters (e.g., MPO, MMP-9), levels of other antioxidants (GSSG, vitamin C, and urate), or enzymes indicative of oxidative stress (HO-1, GST) in BAL, BW, and endobronchial biopsy tissue |
| Health Outcome | Example Reference(s) |
|---|---|
| Emergency department (ED) visits for respiratory diseases, including asthma | [185,186,187,188] |
| Respiratory hospital admissions | [189,190,191,192,193,194] |
| Respiratory physician outpatient visits | [194,195,196,197] |
| Respiratory symptoms | [198,199,200,201] |
| Lung function | [202,203,204,205] |
| Pulmonary and systemic inflammation | [202,206,207] |
| Cardiovascular-related health outcomes | Vascular function- 207; ED visits for cardiovascular diseases-208 a |
| Mortality | [209,210] b |
| As | Cd | Cr | Pb | Hg | Co | Cu | Mn | Ni | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|
| Median (mg/kg) | ||||||||||
| Wood Ash a | 10 | 3.6 | 30.8 | 61.5 | 0 | 9 | 68.2 | 3485 | 16.4 | 329 |
| All Fuels-All Ash fractions b | 9 | 17 | 107.5 | 36 | 9.5 | 16 | 146 | 14,350 | 55 | 1659.5 |
| Wood Chips-All Ash fractions b | 8 | 19 | 132 | 39 | 10 | 14.5 | 180 | 14,366 | 55 | 350 |
| Wood Ash—all boiler types c | 7.98 (558) | 8.4 (619) | 66.4 (567) | 54 (607) | 0.11 (549) | 10.2 (543) | 101 (659) | 8200 (551) | 33 (563) | 1438.5 (656) |
| Waste Wood-fly ash d | 104 | 456 | 404 | 50,000 | <0.5 | 11 | 422 | na | 74 | 164,000 |
| Coal Ash-Fly Ash e | 71 | 1.07 | 133 | 49 | 0.1075 | 7.9 | 140 | 189 | 102 | 152 |
| Coal Ash-Bottom Ash e | 7.2 | <5.5 | 191 | 20 | 0.018 | na | 73 | 262 | 123 | 59 |
| Soil e | 5.8 | 0.2 | 50 | 15 | 0.05 | 7 | 20 | 300 | 15 | 50 |
| All Wood ash—all ash fractions f | 13 (89) | 6.5 (109) | 57.2 (128) | 59 (127) | 0.4 (87) | 9.1 (123) | 97.7 (128) | 7350 (122) | 30 (127) | 1595 (128) |
| Clean wood bottom ash f | <3 (32) | <0.51 (31) | 49 (37) | 15.5 (36) | <0.045 (28) | 7.3 (37) | 59 (37) | 4900 (36) | 20.5 (36) | 400 (37) |
| Clean wood fly ash f | 9.1 (26) | 17 (30) | 54 (31) | 75 (31) | 0.3 (28) | 10 (26) | 120 (31) | 10850 (26) | 31 (31) | 3310 (31) |
| Ash Fraction | Corg. (wt% (d.b.)) | Cl (wt% (d.b.)) | PCDD/F (ng TE/kg d.b.) | PAH (mg/kg d.b.) | B[a]P (µg/kg d.b.) |
|---|---|---|---|---|---|
| Bark combustion | |||||
| Bottom ash | 0.2–0.9 | <0.06 | 0.3–11.7 | 1.4–1.8 | 1.4–39.7 |
| Cyclone fly-ash | 0.4–1.1 | 0.1–0.4 | 2.2–12.0 | 2.0–5.9 | 4.7–8.4 |
| Filter fly-ash | 0.6–4.6 | 0.6–6.0 | 7.7–12.7 | 137.0–195.0 | 900.0–4900.0 |
| Wood chips combustion | |||||
| Bottom ash | 0.2–1.9 | <0.01 | 2.4–33.5 | 1.3–1.7 | 0.0–5.4 |
| Cyclone fly-ash | 0.3–3.1 | 0.1–0.5 | 16.3–23.3 | 27.6–61.0 | 188.0–880.0 |
| Filter fly-ash | − | − | − | − | − |
| Pulverized Wood a Fly Ash | 156 | 1500 | |||
| Sawdust combustion | |||||
| Bottom ash | 0.2–3.4 | <0.1 | 1.3–2.1 | 14.7–21.1 | 21.0–40.5 |
| Cyclone fly-ash | 3.2–15.3 | 0.1–0.6 | 1.5–3.7 | 11.2–150.9 | 180.0–670.0 |
| Filter fly-ash | − | − | − | − | − |
| Straw combustion | |||||
| Bottom ash | 9.0 | 1.1 | 2.3 | 0.1 | 0.0 |
| Cyclone fly-ash | 16.6 | 13.6 | 70.8 | 15.8 | 17.0 |
| Filter fly-ash | 16.1 | 35.1 | 353.0 | 26.0 | 320.0 |
| Cereal combustion | |||||
| Bottom ash | 9.4 | 1.3 | 22.0 | 0.3 | 0.0 |
| Cyclone fly-ash | 9.9 | 5.2 | 12.2 | 0.5 | 0.0 |
| Filter fly-ash | 4.9 | 19.0 | 56.0 | 7.3 | 210.0 |
4.3.4. Respirable Silica
4.3.5. Radioactivity
5. Field Testing at Two Power Stations
5.1. Experience with Biomass Handling at UK Power Plant
5.2. Testing and Analysis of Power Station Exposures
5.2.1. Site Descriptions
5.2.2. Site Testing
5.2.3. Spore Quantification and Identification
5.3. Results and Discussion
5.3.1. Levels of Bacteria and Fungi
5.3.2. Types of Bacteria and Fungi


| Sample | Colony Forming Units /m3 | Genera of Health Significant Fungi Identified | |
|---|---|---|---|
| Bacteria | Fungi | ||
| 1.Screw reclaimer discharge onto conveyor to day silo | 7.3 × 105 | 2.0 × 105 | Mucor spp. Paecilomyces spp. Penicillium spp. Aspergillus spp. Yeast |
| 2. Adjacent to shuttle conveyor, south side | 3.0 × 105 | 7.8 × 105 | Paecilomyces spp. Penicillium spp. Aspergillus spp. |
| 3. Adjacent to fuel input conveyor | 4.6 × 104 | 7.6 × 104 | Paecilomyces spp. Penicillium spp. Aspergillus spp. Yeast |
| 4. Adjacent to shuttle conveyor, north side | 1.42 × 105 | 2.8 × 105 | Paecilomyces spp. Penicillium spp. Yeast |
| Boiler house | <2.0 × 103 | 4.0 × 103 | Paecilomyces spp. Penicillium spp. Mycelia sterilia |
| Adjacent to north side screw reclaimer | 2.2 × 104 | 2.4 × 104 | Paecilomyces spp. Penicillium spp. Aspergillus spp. Yeast |
| Sample | Colony Forming Units/m3 | Genera of Health Significant Fungi Identified | |
|---|---|---|---|
| Bacteria | Fungi | ||
| 1.Screw reclaimer discharge onto conveyor to day silo | <1.00 × 103 | 3.98 × 103 | Paecilomyces spp. Penicillium spp. Yeast |
| 2. Adjacent to shuttle conveyor, south side | 7.94 × 105 | 1.51 × 105 | Mucor spp. Paecilomyces spp. Penicillium spp |
| 3. Adjacent to fuel input conveyor | 2.40 × 105 | 7.76 × 104 | Mucor spp. Penicillium spp. |
| 4. Adjacent to shuttle conveyor, north side | 2.24 × 105 | 7.08 × 104 | Aspergillus spp. Mucor spp. Paecilomyces spp. Penicillium spp. |
| Sample | Colony Forming Units/m3 | Genera of Health Significant Fungi Identified | |
|---|---|---|---|
| Bacteria | Fungi | ||
| 1. Mill bunker floor | 1.51 × 104 | 9.33 × 103 | Aspergillus spp. Cladosporium spp. Mucor spp. Penicillium spp. |
| 2. Transfer tower 2 | <3.98 × 102 | <3.98 × 102 | Aspergillus spp. Mucor spp. Mycelia sterilia Penicillium spp. |
| 3. Transfer tower 1 | <3.98 × 102 | 2.82 × 103 | Mucor spp. Paecilomyces spp. Penicillium spp. Yeast |
| 4. Biomass addition to coal conveyor point | 1.20 × 103 | 4.79 × 103 | Mucor spp. Penicillium spp. |
| 5. Biomass store | <3.98 × 102 | <3.98 × 102 | Mucor spp. Penicillium spp. |
| 6. Coal conveyor prior to biomass addition | <3.98 × 102 | 7.41 × 103 | Aspergillus spp. Penicillium spp. |

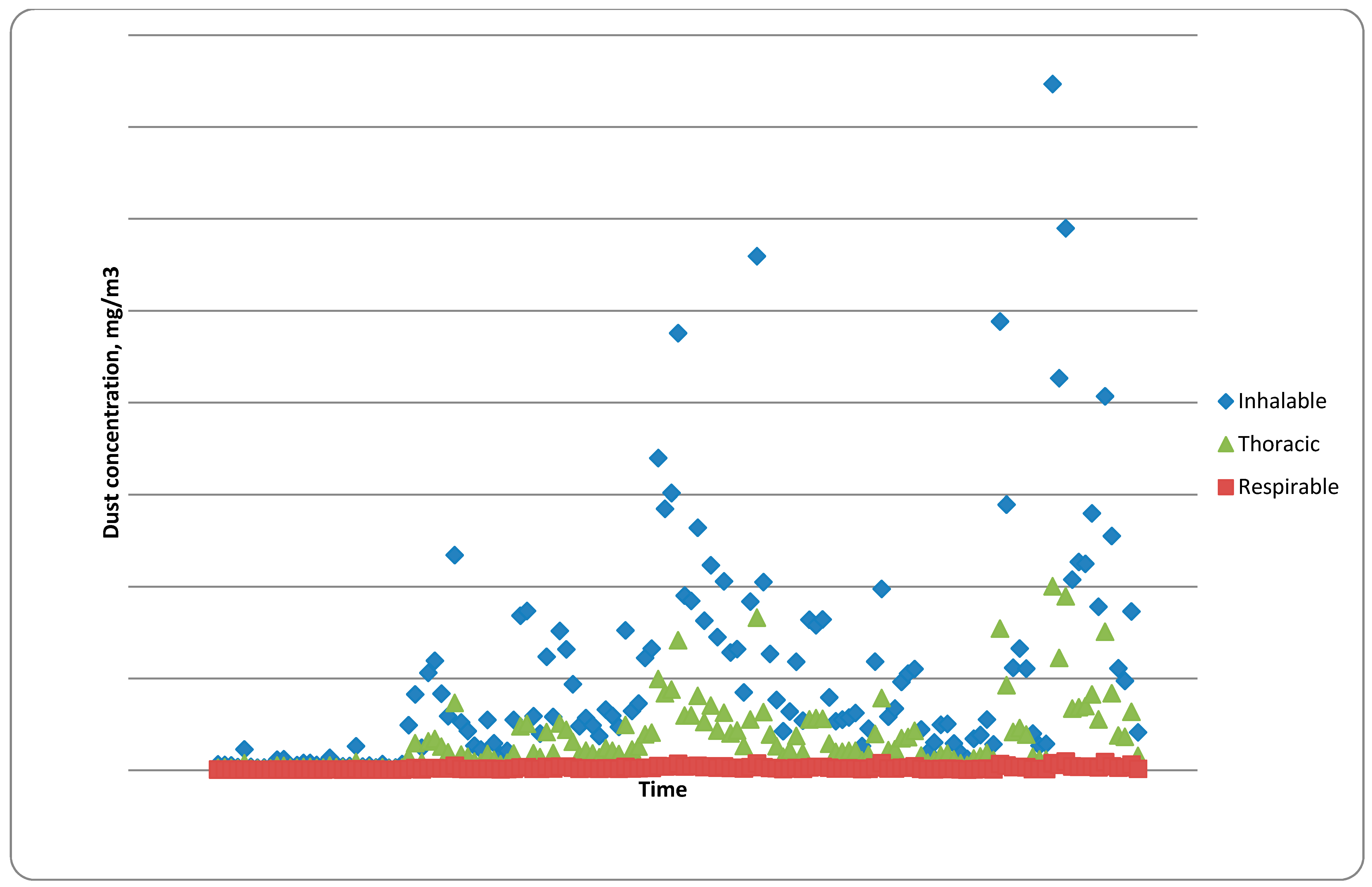
5.3.3. Dust Levels in Plants
| Fungal Group | Health Significance |
|---|---|
| Aspergillus spp. | Common environmental organism being found in soil, plant debris, decaying fruit and vegetables as well as indoor environments. Can act as a potent allergen causing allergic asthma with some species producing mycotoxins. Some species can cause infection in humans invading the lungs, sinuses and other sites sometimes causing deep infections in immunocompromised persons. Non-immunocompromised persons may also occasionally show infection of sinuses and lungs. |
| Mucor spp. | Widespread in soil, plants, decaying vegetation etc. May cause zygomycosis or mucormycosis in humans—infection of nose, septic arthritis, dialysis-associated peritonitis, renal infections, gastritis and lung infections. Exacerbated by persons being immunocompromised or being diabetic |
| Penicillium spp. | Widespread throughout environment especially associated with soil and decaying vegetation. May cause allergic asthma and lead to irritation of respiratory tract. May occasionally cause more serious illness with species capable of producing mycotoxin. |
| Paecilomyces spp. | An inhabitant of soil and decaying vegetation, occasionally found in foods and in air. Often isolated from compost. May give rise to allergic reactions with the immunocompromised most at risk. |
| Yeasts | Common airborne fungus. May be a problem if a person has been previously exposed and has become hypersensitive. High levels may cause allergies. |
| Mycelia sterilia | Ubiquitous with some being important plant pathogens. |
| Cladosporium spp. | Widely distributed in air and rotten organic material and is frequently isolated from foods. Infection may lead to skin lesions, keratitis, nail infections, sinusitis and lung infection. |
| Location number | Continuous Monitor | |
|---|---|---|
| Average inhalable dust level, mg/m3 | Maximum inhalable dust level, mg/m3 | |
| 1 | 0.26 | 0.39 |
| 3 | 0.21 | 0.32 |
| 4 | 0.45 | 1.30 |
| 6 | 0.22 | 0.67 |
| Location number | Continuous Monitor | Static Monitors | |
|---|---|---|---|
| Average inhalable dust level, mg/m3 | Maximum inhalable dust level, mg/m3 | Average inhalable dust level, mg/m3 | |
| 1 | 0.063 | 3.4 | |
| 2 | 0.05 | 1.83 | 1.10 |
| 3 (static monitor) | 0.37 | 2.46 | 0.55 |
| 3 (test team monitor) | 0.15 | 1.59 | |
| 4 | 0.405 | 1.65 | |
| Outside | 0.21 | 6.8 | |
| Location number | Continuous Monitor | Gravimetric Monitor | |
|---|---|---|---|
| Average inhalable dust level, mg/m3 | Maximum inhalable dust level, mg/m3 | Average inhalable dust level, mg/m3 | |
| 1 | 6.10 | 9.64 | |
| 2 | 1.24 | 5.85 | |
| 3 | 1.89 | 3.78 | |
| 4 | 1.98 | 3.71 | |
| 5 (static monitor) | 5.31 | 37.34 | 4.00 |
| 5 (test team monitor) | 0.45 | 1.32 | |
| 6 | 1.58 | 27.66 | |
| Coal plant control room | 0.25 | 0.57 | |
| Outside | 0.41 | 2.11 | |
| Test team gravimetric monitor | 14.23 | ||
| Site | Plant A | Plant B | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Screw reclaimer discharge onto conveyor to day silo | 2. Adjacent to shuttle conveyor, south side | 3. Adjacent to fuel input conveyor | 4. Adjacent to shuttle conveyor, north side | 5. Boiler house | 6. Adjacent to north side screw reclaimer | 1. Mill bunker floor | 2. Transfer tower 2 | 3. Transfer tower 1 | 4. Biomass addition to coal conveyor point | 5. Biomass store | 6. Coal conveyor prior to biomass addition | |||||
| Visit | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | ||||||
| Identified fungal types | ||||||||||||||||
| Mucor spp. | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||
| Paecilomyces spp. | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| Penicillium spp. | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Aspergillus spp. | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| Yeast | √ | √ | √ | √ | √ | √ | ||||||||||
| Mycelia sterilia | √ | √ | ||||||||||||||
| Cladosporium spp. | √ | |||||||||||||||

5.4. Conclusions of Field Sampling
6. Conclusions
Author Contributions
Conflicts of Interest
References
- McKendry, P. Energy production from biomass (Part I): Overview of biomass. Bioresour. Tech. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Biomass Combined Heat and Power Catalog of Technologies. Combined Heat and Power Partnership; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2007.
- Congressional Research Service (CRS). Biomass Feedstock for Biopower: Background and Selected Issues. CRS Report for Congress; CRS 7–5700. R41440; Congressional Research Service (CRS): Washington, DC, USA, 2010.
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, E. Bioaerosol health effects and exposure assessment progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, K.; Axelsson, S.; Arvidsson, H.; Ing-Liss, B.; Lundholm, C.; Eriksson, K. Exposure to wood dust, resin acids, and volatile organic compounds during production of wood pellets. J. Occup. Environ. Hyg. 2008b, 5, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Babcock & Wilcox Power Generation Group (2008) Brochure E101–3161A. Available online: http://www.babcock.com/library/Documents/e1013161.pdf (accessed on 13 May 2011).
- International Finance Corporation (IFC). Environmental, Health and Safety Guidelines. Thermal Power Plants; International Finance Corporation (IFC): Washington, DC, USA, 2008. [Google Scholar]
- Van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008. [Google Scholar]
- ECN. Phyllis2, Database for Biomass and Waste, Energy Research Centre of the Netherlands 2012. Available online: https://www.ecn.nl/phyllis2 (accessed on 13 May 2011).
- Directive 2010/75/EU of The European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and control). Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:334:0017:0119:EN:PDF (accessed on 13 May 2011).
- Oakridge National Laboratory. Biomass Energy Data Book; US Department of Energy (DOE): Washington, DC, USA, 2006.
- Best Available Techniques (BAT) Reference Document for the Large Combustion Plants (Currently under Review; Latest Working Draft and Previous Version. 2013. Available online: http://eippcb.jrc.ec.europa.eu/reference/ (accessed on 13 May 2011).
- Parikh, J.; Channiwalla, S.A.; Ghosal, G.K. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Elliott, D.C.; Peterson, K.L.; Muzatko, D.S.; Alderson, E.V.; Hart, T.R.; Neuenschwander, G.G. Effects of trace contaminants on catalytic processing of biomass-derived feedstocks. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; Humana Press: New York, NY, USA, 2004. [Google Scholar]
- Nordin, A. Chemical elemental characteristics of biomass fuels. Biomass Bioenergy 1994, 6, 339–347. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical characterization and chemical composition of densified biomass fuels with regard to their combustion behavior. Biomass Bioenergy 2004, 24, 51–57. [Google Scholar]
- Madsen, A.M.; Sharma, A.K. Sampling of high amounts of bioaerosols using a high-volume electrostatic field sampler. Ann. Occup. Hyg. 2008, 52, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M. Airborne endotoxin in different background environments and seasons. Ann. Agric. Environ. Med. 2006a, 13, 81–86. [Google Scholar]
- Madsen, A.M. Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann. Occup. Hyg. 2006b, 50, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Madsen, A.M.; Martensson, L.; Pomorska, D.; Larsson, L. Assessment of microbial exposure risks from handling of biofuel wood chips and straw—Effect of outdoor storage. Ann. Agric. Environ. Med. 2006, 13, 139–145. [Google Scholar] [PubMed]
- Miles, T.R.; Mile, T.R., Jr.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Davis, C.A.; Oden, L.L. Alkali Deposits Found in Biomass Power Plants. National Renewable Energy Laboratory Report; National Renewable Energy Laboratory: Golden, CO, USA, 1995.
- Cohn, C.A.; Lemieux, C.L.; Long, A.S.; Kystol, J.; Vogel, U.; White, P.A.; Madsen, A.M. Physical-chemical and microbiological characterization, and mutagenic activity of airborne PM sampled in a biomass-fueled electrical production facility. Environ. Mol. Mutagen. 2011, 52, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, K.; Lundholm, C.; Eriksson, K.; Liljelind, I. Variability and determinants of wood dust and resin acid exposure during wood pellet production measurement strategies and bias in assessing exposure-response relationships. Ann. Occup. Hyg. 2008, 52, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Martensson, L.; Schneider, T.; Larsson, L. Microbial dustiness and particle release of different biofuels. Ann. Occup. Hyg. 2004, 48, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Edman, K.; Lofstedt, H.; Berg, P.; Eriksson, K.; Axelson, S.; Bryngelsson, I.; Federli, C. Exposure assessment to α- and β-pinene, Δ3-carene and wood dust in industrial production of wood pellets. Ann. Occup. Hyg. 2003, 47, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Shankar, T.J.; Sokhansanj, S.; Lim, C.J.; Bi, X.T.; Melin, S. Effects of headspace and oxygen level on off-gas emission from wood pellets in storage. Ann. Occup. Hyg. 2009, 53, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, U.; Hogberg, H.E.; Hogberg, J.; Galle, G. Emission of hexanal and carbon monoxide from storage of wood pellets, a potential occupational and domestic health hazard. Ann. Occup. Hyg. 2004, 48, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, U.; Samuelsson, J.; Melin, S. Hazardous off-gassing of carbon monoxide and oxygen depletion during ocean transportation of wood pellets. Ann. Occup. Hyg. 2008, 52, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Hildegard, G.; Lory, M.; Kramer, T.; Thali, M.; Bartsch, C. Lethal carbon monoxide poisoning in wood pellet store rooms—Two cases and a review of the literature. Ann. Occup. Hyg. 2012, 56, 755–763. [Google Scholar] [CrossRef] [PubMed]
- AP 42, Compilation of Air Pollutant Emission Factors; US Environmental Protection Agency (EPA): Washington, DC, USA, 2003; Chapter 1.6.
- Renewable and Alternative Energy Fact Sheet: Co-Firing Biomass with Coal; College of Agricultural Sciences, Pennsylvania State University: State College, PA, USA, 2010.
- Johansson, L.S.; Tullin, C.; Leckner, B.; Sjovall, P. Particle emissions from biomass combustion in small combustors. Biomass Bioenergy 2003, 25, 435–446. [Google Scholar] [CrossRef]
- Wielgosinski, G. The reduction of dioxin emissions from the processes of heat and power generation. J. Air Waste Manag. Assoc. 2011, 61, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, T. Combustion and co-combustion of biomass: Fundamentals, technologies, and primary measures for emission reduction. Energy Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- Combustion in Europe: Overview on Technologies and Regulations (Final); Report prepared for New York State Energy Research and Development Agency (NYSERDA): Albany, NY, USA, 2008.
- Rosenberg, C.; Liukkonen, T.; Kallas-Tarpila, T.; Ruonakangas, A.; Ranta, R.; Nurminen, M.; Welling, I.; Jäppinen, P. Monoterpene and wood dust exposures; work-related symptoms among Finnish sawmill workers. Am. J. Ind. Med. 2002, 41, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Demers, P.A.; Teschke, K.; Davies, H.W.; Kennedy, S.M.; Leung, V. Exposure to dust, resin acids, and monoterpenes in softwood lumber mills. Am. Ind. Hyg. Assoc. J. 2000, 61, 521–528. [Google Scholar]
- Eriksson, K.A.; Stjernberg, N.L.; Levin, J.O.; Hammarström, U.; Ledin, M.C. Terpene exposure and respiratory effects among sawmill workers. Scand. J. Work Environ. Health 1996, 22, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.A.; Levin, J.O.; Sandström, T.; Lindström-Espeling, K.; Lindén, G.; Stjernberg, N.L. Terpene exposure and respiratory effects among workers in Swedish joinery shops. Scand. J. Work Environ. Health 1997, 23, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jappinen, P.; Haahtela, T.; Lira, J. Chip pile workers and mould exposure. Allergy 1987, 42, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Van Assendelft, A.H.; Raitio, M.; Turkia, V. Fuel chip induced hypersensitivity pneumonitis caused by Penicillium species. Chest 1985, 87, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Thornqvist, T.; Lundstrom, H. Health hazards caused by fungi in stored wood chips. For. Products J. 1982, 32, 29–32. [Google Scholar]
- Eriksson, K.; Hagström, K.; Axelsson, S.; Nylander-French, L. Tape-stripping as a method for measuring dermal exposure to resin acids during wood pellet production. J. Environ. Monit. 2008, 10, 345–352. [Google Scholar] [CrossRef] [PubMed]
- TLVs and BEIs: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. In Proceedings of American Conference of Governmental Industrial Hygienists, Cincinnati, USA; 2011.
- ToxFAQ for Carbon Monoxide. Available online: http://www.atsdr.cdc.gov/toxfaqs/tfacts201.pdf (accessed on 16 December 2011).
- ToxFAQ for Nitrogen Oxides (Nitric Oxide, Nitrogen Dioxide, etc.). Available online: http://www.atsdr.cdc.gov/toxfaqs/tfacts175.pdf (accessed on 16 December 2011).
- ToxFAQs for Sulfur Dioxide. Available online: www.atsdr.cdc.gov/tfacts116.pdf (accessed on 16 December 2011).
- ToxFAQ for Sulfur Trioxide (SO3) and Sulfuric Acid. Available online: http://www.atsdr.cdc.gov/tfacts117.pdf (accessed on 16 December 2011).
- ToxFAQ for 1,3-Butadiene. Available online: http://www.atsdr.cdc.gov/toxfaqs/tfacts28.pdf (accessed on 16 December 2011).
- ToxFAQ for Polycyclic Aromatic Hydrocarbons (PAHs). Available online: http://www.atsdr.cdc.gov/tfacts69.pdf (accessed on 16 December 2011).
- ToxFAQ for Benzene. Available online: http://www.atsdr.cdc.gov/tfacts3.pdf (accessed on 16 December 2011).
- ToxFAQ for Formaldehyde. Available online: http://www.atsdr.cdc.gov/tfacts111.pdf (accessed on 16 December 2011).
- Occupational Health Guide for Methyl Alcohol. Available online: http://www.cdc.gov/niosh/docs/81-123/pdfs/0397.pdf (accessed on 15 December 2011).
- ToxFAQ for Cresols. Available online: http://www.atsdr.cdc.gov/tfacts34.pdf (accessed on 16 December 2011).
- Occupational Health Guide for Hydroquinone. Available online: http://www.cdc.gov/niosh/docs/81-123/pdfs/0338.pdf (accessed on 16 December 2011).
- Material Safety Data Sheet for 9-Fluorenone. Available online: http://www.sciencelab.com/msds.php?msdsId=9924071 (accessed on 15 December 2011).
- Material Safety Data Sheet for Anthraquinone, 97%. Available online: http://fscimage.fishersci.com/msds/97262.htm (accessed on 15 December 2011).
- IRIS Record for Dichloromethane [methylene chloride] (CASRN 75-09-2). Available online: http://www.epa.gov/iris/subst/0070.htm (accessed on 15 December 2011).
- Methylene Chloride. In NIOSH Pocket Guide to Chemical Hazards. Available online: http://www.cdc.gov/niosh/npg/npgd0414.html (accessed on 15 December 2011).
- ToxFAQ for Chloromethane (CAS #74-87-3). Available online: http://www.atsdr.cdc.gov/tfacts106.pdf (accessed on 16 December 2011).
- ToxFAQ for Chlorinated Dibenzo-p-Dioxins (CDDs). Available online: http://www.atsdr.cdc.gov/tfacts104.pdf (accessed on 16 December 2011).
- Toxicological Profile for Chlorinated Dibenzo-p-dioxins; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 1998.
- Particle Pollution and Your Health. Available online: http://www.epa.gov/air/particlepollution/pdfs/pm-color.pdf (accessed on 15 December 2011).
- IRIS Record for Diesel Engine Emissions. Available online: http://www.epa.gov/IRIS/subst/0642.htm (accessed on 16 December 2011).
- ToxFAQ for Aluminum. Available online: http://www.atsdr.cdc.gov/tfacts22.pdf (accessed on 16 December 2011).
- ToxFAQ for Arsenic. Available online: http://www.atsdr.cdc.gov/tfacts2.pdf (accessed on 16 December 2011).
- ToxFAQ for Beryllium. Available online: http://www.atsdr.cdc.gov/tfacts33.pdf (accessed on 16 December 2011).
- IRIS Record for Beryllium and Compounds. Available online: http://www.epa.gov/iris/subst/0012.htm (accessed on 15 December 2011).
- ToxFAQ for Cobalt. Available online: http://www.atsdr.cdc.gov/tfacts33.pdf (accessed on 16 December 2011).
- Material Safety Data Sheet for Magnesium Oxide. Available online: http://fscimage.fishersci.com/msds/13450.htm (accessed on 16 December 2011).
- Material Safety Data Sheet for Iron. Available online: http://fscimage.fishersci.com/msds/11490.htm (accessed on 16 December 2011).
- ToxFAQ for Manganese. Available online: http://www.atsdr.cdc.gov/tfacts151.pdf (accessed on 16 December 2011).
- IRIS Record for Manganese. Available online: http://www.epa.gov/iris/subst/0373.htm (accessed on 15 December 2011).
- Agency for Toxic Substances and Disease Registry (ATSDR) August 2005. ToxFAQ for Zinc (CAS #7439-66-6). Available online: http://www.atsdr.cdc.gov/tfacts60.pdf (accessed on 16 December 2011).
- IRIS Record for Zinc. Available online: http://www.epa.gov/iris/subst/0426.htm (accessed on 15 December 2011).
- ToxFAQ for Nickel. Available online: http://www.atsdr.cdc.gov/tfacts15.pdf (accessed on 16 December 2011).
- IRIS Record for Nickel Subsulfide. Available online: http://www.epa.gov/iris/subst/0273.htm (accessed on 15 December 2011).
- ToxFAQ for Copper. Available online: http://www.atsdr.cdc.gov/tfacts132.pdf (accessed on 16 December 2011).
- ToxFAQs Sheet for Lead. Available online: http://www.atsdr.cdc.gov/tfacts13.htm (accessed on 3 March 2000).
- IRIS Record for Mercury, Elemental. Available online: http://www.epa.gov/iris/subst/0370.htm (accessed on 15 December 2011).
- ToxFAQ for Chromium. Available online: http://www.atsdr.cdc.gov/tfacts7.pdf (accessed on 16 December 2011).
- ToxFAQ for Cadmium (CAS #7440-43-9). Available online: http://www.atsdr.cdc.gov/tfacts5.pdf (accessed on 16 December 2011).
- Occupational Health Guide for Cadmium Dust (as Cadmium). Available online: http://www.cdc.gov/niosh/docs/81-123/pdfs/0087.pdf (accessed on 15 December 2011).
- Occupational Health Guide for Crystalline Silica. Available online: http://www.cdc.gov/niosh/docs/81-123/pdfs/0553.pdf (accessed on 15 December 2011).
- Kolmodin-Hedman, B.; Blomquist, G.; Lofgren, F. Chipped wood as a source of mould exposure. Eur. J. Resp. Dis. 1987, 71, 44–51. [Google Scholar]
- Alwis, K.U.; Mandryk, J.; Hoching, A.D. Exposure to biohazards in wood dust: Bacteria, fungi, endotoxins, and (1,3) β-d-glucans. Appl. Occup. Environ. Hyg. 1999, 14, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.; Graneek, B.J.; Turner-Warwick, M.; Taylor, A.J. Extrinsic allergic alveolitis and asthma in a sawmill worker: Case report and review of the literature. Occup. Environ. Med. 1994, 51, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Ławniczek-Wałczyk, A.; Gołofit-Szymczak, M.; Cyprowski, M.; Górny, R.L. Exposure to harmful microbiological agents during the handling of biomass for power production purposes. Med. Pr. 2012, 63, 395–407. [Google Scholar] [PubMed]
- Polish Ministry of Health. Ordinance issued by the Minister of Health on 22 April 2005 on occupational biological hazards and health protection of people occupationally exposed to such hazards. J. Law. 2005. (In Polish) [Google Scholar]
- Zock, J.P.; Hollander, A.; Heederik, D.; Douwes, J. Acute lung function changes and low endotoxin exposures in the potato processing industry. Amer. J. Ind. Med. 1998, 33, 384–391. [Google Scholar] [CrossRef]
- Rongo, L.M.; Msamanga, G.I.; Burstyn, I.; Barten, F.; Dolmans, W.M.; Heederik, D. Exposure to wood dust and endotoxin in small-scale wood industries in Tanzania. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Eduard, W. Fungal spores: A critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol. 2009, 39, 799–864. [Google Scholar] [CrossRef] [PubMed]
- Oppliger, A.; Rusca, S.; Charriere, N. Assessment of bioaerosols and inhalable dust exposure in Swiss sawmills. Ann. Occup. Hyg. 2005, 49, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Schlunssen, V.; Olsen, T.T.; Sigsgaard, T.; Avci, H. Airborne fungal and bacterial components in PM1 dust from biofuel plants. Ann. Occup. Hyg. 2009, 53, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J. (1→3)-β-d-glucans and respiratory health: A review of the scientific evidence. Indoor Air 2005, 15, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Health-Based Recommended Occupational Exposure Limit; Health Council of the Netherlands. The Hague: Health Council of the Netherlands. 2010. Available online: http://www.gr.nl/en/publications/healthy-working-conditions/endotoxins-health-based-recommended-occupational-exposure-li (accessed on 13 May 2011).
- Wouters, I.M.; Spann, S.; Douwes, J.; Doekes, G.; Heederik, A. Overview of personal occupational exposure levels to inhalable dust, endotoxin, β(1→3)-Glucan and fungal extracellular polysaccharides in the waste management chain. Ann. Occoup. Hyg. 2006, 50, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Saber, A.T.; Nordly, P.; Sharma, A.K.; Wallin, H.; Vogel, U. Inflammation but no DNA (deoxyribonucleic acid) damage in mice exposed to airborne dust from a biofuel plant. Scand. J. Work Environ. Health 2008b, 34, 278–287. [Google Scholar] [CrossRef]
- Shibuya, K.; Paris, S.; Ando, T.; Nakayama, H.; Hatori, T.; Latge, J.P. Catalases of Aspergillus fumigatus and inflammation in aspergillosis. Nippon Ishinkin Gakkai Zasshi 2006, 47, 249–255. [Google Scholar] [CrossRef]
- Jacob, B.; Ritz, B.; Gehring, U.; Koch, A.; Bischof, W.; Wichmann, H.E.; Heinrich, J. Indoor exposure to molds and allergic sensitization. Environ. Health Perspect. 2002, 110, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Schlunssen, V.; Madsen, A.M.; Skov, S.; Sigsgaard, T. Does the use of biofuels affect respiratory health among male Danish energy plant workers? Occup. Environ. Med. 2011, 68, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Liljelind, I. Consensus report for wood dust. In Scientific Basis for Swedish Occupational Standards; National Institute of Working Life: Stockholm, Sweden, 2000; pp. 51–71. [Google Scholar]
- International Agency Research Cancer (IARC). Wood dust and formaldehyde. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemical to Humans; IARC: Lyon, France, 1995; Volume 62. [Google Scholar]
- HSE (UK Health and Safety Executive) Toxic Woods, HSE Information Sheet: Woodworking Sheet No. 30, 2012a. Available online: http://www.hse.gov.uk/pubns/wis30.pdf (accessed on 13 May 2011).
- 2014 TLVs and BEIs; American Association of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2014.
- Directive 1999/38/EC Amending for the Second Time Directive 90/394/EEC on the Protection of Workers from the Risks Related to Exposure to Carcinogens at Work and Extending It to Mutagens 1999. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:01999L0038–19990601&rid=1 (accessed on 13 May 2011).
- Kauppinen, T.; Vincent, R.; Liukkonen, T.; Grzebyk, M.; Kauppinen, A.; Welling, I.; Arezes, P.; Black, N.; Bochmann, F.; Campelo, F.; et al. Occupational exposure to inhalable wood dust in the member states of the European Union. Ann. Occup. Hyg. 2006, 50, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Levin, J.O. Identification of cis- and trans-verbenol in human urine after occupational exposure to terpenes. Int. Arch. Occup. Environ. Health 1990, 62, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, R. Malignant disease of the paranasal sinuses. J. Larngol. Otol. 1965, 79, 592–612. [Google Scholar] [CrossRef]
- Acheson, E.D.; Cowdell, R.H.; Hadfield, E.; Macbeth, R.G. Nasal cancer in woodworkers in the furniture industry. Brit. Med. J. 1968, 2, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Klintenberg, C.; Olofsson, J.; Hellquist, H.; Sökjer, H. Adenocarcinoma of the ethmoid sinuses: A review of 28 cases with special reference to wood dust exposure. Cancer 1984, 54, 482–488. [Google Scholar] [CrossRef]
- Hayes, R.B.; Gerin, M.; Raatgever, J.W.; de Bruyn, A. Wood-related occupations, wood dust exposure, and sinonasal cancer. Amer. J. Epidemiol. 1986, 124, 569–577. [Google Scholar]
- Olsen, J.H.; Asnaes, S. Formaldehyde and the risk of squamous cell carcinoma of the sinonasal cavities. Brit. J. Ind. Med. 1986, 43, 769–774. [Google Scholar] [CrossRef]
- Luce, D.; Leclerc, A.; Morcet, J.F.; Casallareo, A.; Gerin, M.; Brugere, J.; Haguenoer, M.; Goldberg, M. Occupational risk factors for sinonasal cancer—A case-control study in France. Amer. J. Ind. Med. 1992, 21, 163–175. [Google Scholar] [CrossRef]
- Luce, D.; Gérin, M.; Leclerc, A.; Morcet, J.F.; Brugére, J.; Goldber, M. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int. J. Cancer 1993, 53, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; Chow, W.-H.; McLaughlin, J.K. Wood dust and nasal cancer risk. J. Occup. Environ. Med. 1997, 39, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.O.; Brandt, K.; Peek, R.D.; Schmitt, U. Quantitative measurement and chemical analysis of wood dust collected in German woodworking companies. Holz. Roh- Werkstoff 1997, 55, 141–147. [Google Scholar] [CrossRef]
- Stellman, S.D.; Demers, P.A.; Colin, D.; Bofetta, P. Cancer mortality and wood dust exposure among participants in the American Cancer Prevention Study-II (CPS-II). Amer. J. Ind. Med. 1998, 34, 229–237. [Google Scholar] [CrossRef]
- Barcenas, C.H.; Delclos, G.L.; el-Zein, R.; Tortolero-Luna, G.; Whitehead, L.W.; Spitz, M.R. Wood dust exposure and the association with lung cancer risk. Amer. J. Ind. Med. 2005, 47, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, V.; Natarajan, K.K.; Moysich, K.B.; Rigual, N.R.; Ramnath, N.; Natarajan, N.; Reid, M.E. Wood dust exposure and risk of upper aero-digestive and respiratory cancers in males. Occup. Environ. Med. 2008, 65, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Ordman, D. Bronchial asthma caused by the inhalation of wood dust. Ann. Allergy 1949, 7, 492–496. [Google Scholar] [PubMed]
- De Zotti, R.; Gubian, F. Asthma and rhinitis in wooding workers. Allergy Asthma Proceed. 1996, 17, 199–203. [Google Scholar] [CrossRef]
- Fernández-Rivas, M.; Pérez-Carral, C.; Senent, C.J. Occupational asthma and rhinitis caused by ash (Fraxinus excelsior) wood dust. Allergy 1997, 52, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Mandryk, J.; Alwis, K.U.; Hocking, A.D. Work related symptoms and dose-response relationships for personal exposures and pulmonary function among wood workers. Amer. J. Ind. Med. 1999, 35, 481–490. [Google Scholar] [CrossRef]
- Mandryk, J.; Alwis, K.U.; Hocking, A.D. Effects of personal exposure on pulmonary function and work-related symptoms among sawmill workers. Ann. Occup. Hyg. 2000, 44, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rios, M.; Ruano-Ravina, A.; Etminan, M.; Takkouche, B. A meta-analysis on wood dust exposure and risk of asthma. Allergy 2010, 65, 467–473. [Google Scholar] [CrossRef] [PubMed]
- HSE (UK Health and Safety Executive) THOR—Voluntary Reporting of Occupational Diseases by Specialist Doctors: Index of THOR Tables. “Table THORR06; Occupational Asthma: Estimated Number of Diagnoses in Which Particular Causative Substances were Identified. Reported by Chest physicians to SWORD between 1998 and 2012”. Available online: http://www.hse.gov.uk/statistics/tables/index.htm#thor (accessed on 13 May 2011).
- Rohr, A.C. The health significance of gas- and particle-phase terpene oxidation products: A review. Environ. Int. 2013, 60, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Keira, T.; Sundgren, M.; Levin, J.O.; Eriksson, K.; Carlson, R. Adverse effects of colophony. Ind. Health 1997, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- ECHA Database on Registered Chemicals. Available online: http://echa.europa.eu/information-on-chemicals/registered-substances (accessed on 15 July 2015).
- Ernstgård, L.; Iregren, A.; Sjögren, B.; Svedberg, U.; Johanson, G. Acute effects of exposure to hexanal vapors in humans. J. Occup. Environ. Med. 2006, 46, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Melin, S. Review of Off-Gassing from Wood Pellets—A Canadian Perspective; Wood Pellet Association of Canada: Revelstoke, Canada, 2010. [Google Scholar]
- HSE (UK Health and Safety Executive) Risk of Carbon Monoxide Release during the Storage of Wood Pellets. HSE Safety Notice OPSTD 3 November 2012. Available online: http://www.hse.gov.uk/safetybulletins/co-wood-pellets.htm (accessed on 13 May 2011).
- Kuang, X.; Shankar, T.J.; Sokhansanj, S.; Bi, X.T. Characterization and kinetics study of off gas emission from stored wood pellets. Ann. Occup. Hyg. 2008, 53, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Gref, F. Emission of volatile organic compounds from softwood pellets during storage. For. Prod. J. 2005, 55, 132–135. [Google Scholar]
- He, X.; Lau, A.K.; Sokhansanj, S.; Lin, C.J.; Bi, X.T.; Melin, S. Dry matter losses in combination with gaseous emissions during the storage of forest residues. Fuel 2012, 95, 662–664. [Google Scholar] [CrossRef]
- Boman, B.C.; Forsberg, A.B.; Jarvholm, B.G. Adverse health effects from ambient air pollution in relation to residential wood combustion in modern society. Scand. J. Work Environ. Health 2003, 29, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zelikoff, J.T.; Chen, L.C.; Cohen, M.D.; Schlesinger, R.B. The toxicology of inhaled wood smoke. J. Toxicol. Environ. Health B Crit. Rev. 2002, 5, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Kocbach Bølling, A.; Pagels, J.; Yttri, K.E.; Barregard, L.; Sallsten, G.; Schwarze, P.E.; Boman, C. Health effects of residential wood smoke particles: The importance of combustion conditions and physicochemical particle properties. Part. Fibre. Toxicol. 2009. [Google Scholar] [CrossRef] [PubMed]
- United Nations Economic Commission for Europe (UN ECE). Recent Results and Updating of Scientific and Technical Knowledge: Health Risks of Air Pollution from Biomass Combustion. Report by the Task Force on Health, Executive Body for the Convention on Long-range Transboundary Air Pollution, ECE/EB.AIR/WG.1/2009/12; United Nations Economic Commission for Europe (UN ECE): Geneva, Switzerland, 2009; p. 12. [Google Scholar]
- Wegesser, T.C.; Pinkerton, K.E.; Last, J.A. California wildfires of 2008: Coarse and fine particulate matter toxicity. Environ. Health Perspect. 2009, 117, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W.; Long, C.M.; Bunn, W.B.; Sax, S.N.; Lapin, C.A.; Valberg, P.A. Non-cancer health effects of diesel exhaust: A critical assessment of recent human and animal toxicological literature. Crit. Rev. Toxicol. 2009, 39, 195–227. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Household Use of Solid Fuels and High-temperature Frying. In IARC Monographs on the Evaluations of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 95. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Integrated Science Assessment for Particulate Matter (Final). Office of Research and Development, National Center for Environmental Assessment (NCEA)—RTP Division, EPA/600/R-08/139F; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2009; p. 2228.
- Klippel, V.; Nussbaumer, T. Health Relevance of Particles from Wood Combustion in Comparison to Diesel Soot. In Proceedings of the 15th European Biomass Conference and Exhibition, Berlin, Germany, 7–11 May 2007.
- Turner, M.C.; Krewski, D.; Pope CA, I.I.I.; Chen, Y.; Gapstur, S.M.; Thun, M.J. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never smokers. Amer. J. Respir. Crit. Care Med. 2011, 184, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Rohr, A.C.; Wyzga, R.E. Attributing health effects to individual particulate matter constituents. Atmos. Environ. 2012, 62, 130–152. [Google Scholar] [CrossRef]
- Janssen, N.; Hoek, G.; Simic-Lawson, M.; Fischer, P.; van Bree, L.; ten Brink, H.; Keuken, M.; Atkinson, R.W.; Anderson, H.R.; Brunekreef, B.; et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Lippmann, M.; Chen, L.C. Effects of metals within ambient air particulate matter (PM) on human health. Inhal. Toxicol. 2009, 21, 1–31. [Google Scholar]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manag. Assoc. 2000, 50, 1565–1618. [Google Scholar] [CrossRef] [PubMed]
- Morandi, M.T.; Ward, T.J. Wood smoke risk assessment: Defining the questions. Inhal. Toxicol. 2010, 22, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Mar, T.F.; Ito, K.; Koenig, J.Q.; Larson, T.V.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Neas, L.; et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. Expo. Sci. Environ. Epidemiol. 2006, 16, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kocbach, A.; Herseth, J.I.; Låg, M.; Refsnes, M.; Schwarze, P.E. Particles from wood smoke and traffic induce differential pro-inflammatory response patterns in co-cultures. Toxicol. Appl. Pharmacol. 2008, 232, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Myatt, T.A.; Vincent, M.S.; Kobzik, L.; Naeher, L.P.; MacIntosh, D.L.; Suh, H. Markers of inflammation in alveolar cells exposed to fine particulate matter from prescribed fires and urban air. J. Occup. Environ. Med. 2011, 53, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.D.; Mundt, K.A.; Luippold, R.S.; Nunes, A.P.; Cohen, L.; Burch, M.T.; Heidenreich, M.J.; Bachand, A.M. A cohort mortality study of employees in the U.S. carbon black industry. J. Occup. Environ. Med. 2006, 48, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Fryzek, J.P.; Chadda, B.; Marano, D.; White, K.; Schweitzer, S.; McLaughlin, J.K.; Blot, W.J. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J. Occup. Environ. Med. 2003, 45, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Soutar, A.; Cherrie, J.W.; Granath, F.; Andersen, A.; Anttila, A.; Blettner, M.; Gaborieau, V.; Klug, S.J.; Langard, S.; et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control 2004, 15, 697–706. [Google Scholar] [CrossRef] [PubMed]
- EPRI. Fly Ash Exposure in Coal-Fired Power Plants. Electric Power and Research Institute, Report Number TR-102576; EPRI: Palo Alto, CA, USA, 1993. [Google Scholar]
- Hicks, J.; Yager, J. Airborne crystalline silica concentrations at coal-fired power stations associated with coal fly ash. J. Occup. Environ. Hyg. 2006, 3, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, R.J.; te Winkel, H.; Stam, A.F. Environmental and health aspects of coal and biomass co-combustion ashes. In Proceedings of the World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011; p. 11.
- Borm, P.J. Toxicity and occupational health hazards of coal fly ash (CFA). A review of data and comparison to coal mine dust. Ann. Occup. Hyg. 1997, 41, 659–676. [Google Scholar] [CrossRef] [PubMed]
- EPRI. PCDDs and PCDFs in Coal Combustion By-Products (CCBS). Electric Power and Research Institute, Report Number TR-110399; EPRI: Palo Alto, CA, USA, 1998. [Google Scholar]
- Bradley, L.J.N.; Perry, A.E.; Vosnakis, K.A.S.; Archer, C. PAHs and dioxins not present in fly ash at levels of concern. In Proceedings of the World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 March 2013.
- Pitman, R.M. Wood ash use in forestry—A review of the environmental impacts. Forestry 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Kassman, H.; Bäver, L.; Åmand, L.E. The importance of SO2 and SO3 for sulphation of gaseous KCl—An experimental investigation in a biomass fired CFB boiler. Comb. Flame 2010, 157, 1649–1657. [Google Scholar] [CrossRef]
- ECHA. European Chemical Agency Dossier on Ashes (Residues), Plant. 2010. Available online: http://apps.echa.europa.eu/registered/data/dossiers/DISS-9875c4a1-ac42–5c3b-e044–00144f67d031/DISS-9875c4a1-ac42–5c3b-e044–00144f67d031_DISS-9875c4a1-ac42–5c3b-e044–00144f67d031.html (accessed on 13 May 2011).
- SLU. Swedish University of Agricultural Sciences Wood Ash Database. 2014. Available online: http://woodash.slu.se/eng/ (accessed on 13 May 2011).
- Someshwar, A.V. Wood and combination wood-fired boiler ash characterization. J. Environ. Qual. 1996, 25, 962–972. [Google Scholar] [CrossRef]
- Meij, R.; te Winkel, H. The emissions of heavy metals and persistent organic pollutants from modern coal-fired power stations. Atmos. Environ. 2007, 41, 9262–9272. [Google Scholar] [CrossRef]
- Jokiniemi, J.; Hytonen, K.; Tissari, J.; Obernberger, I; Brunner, T; Barnthaler, G.; et al. Biomass Combustion in Residential Heating: Particulate Measurements, Sampling, and Physicochemical and Toxicological Characterization; Final report; University of Kuopio, Fine Particle Aerosol Technology Laboratory, August 2008. [Google Scholar]
- Douben, P.E.T. PAHs: An Ecotoxicological Perspective; Douben, P.E.T., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Sarenbo, S. Wood ash dilemma-reduced quality due to poor combustion performance. Biomass Bioenergy 2009, 33, 1212–1220. [Google Scholar] [CrossRef]
- Bradley, L.J.N.; Magee, B.H.; Allen, S.L. Background levels of polycyclic aromatic hydrocarbons (PAH) and selected metals in New England urban soils. J. Soil Contam. 1994, 3, 349–361. [Google Scholar]
- Barregard, L.; Sällsten, G.; Gustafson, P.; Andersson, L.; Johansson, L.; Basu, S.; Stigendal, L. Experimental exposure to wood-smoke particles in healthy humans: Effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal. Toxicol. 2006, 18, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Sällsten, G.; Andersson, L.; Almstrand, A.C.; Gustafson, P.; Andersson, M.; Olin, A.C. Experimental exposure to wood smoke: Effects on airway inflammation and oxidative stress. Occup. Environ. Med. 2008, 65, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Bräuner, E.V.; Barregard, L.; Sällsten, G.; Wallin, M.; Olinski, R.; Rozalski, R.; Møller, P.; Loft, S. Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutat. Res. 2008, 642, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sällsten, G.; Gustafson, P.; Johansson, L.; Johannesson, S.; Molnár, P.; Strandberg, B.; Tullin, C.; Barregard, L. Experimental wood smoke exposure in humans. Inhal. Toxicol. 2006, 18, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Soukup, J.M.; Case, M.; Dailey, L.A.; Richards, J.; Berntsen, J.; Devlin, R.B.; Stone, S.; Rappold, A. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup. Environ. Med. 2012, 69, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Hansen, J.C.; Kuprov, R.; Sanders, M.D.; Anderson, M.N.; Eatough, D.J. Vascular function and short-term exposure to fine particulate air pollution. J. Air Waste Manag. Assoc. 2011, 61, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Riddervold, I.S.; Bønløkke, J.H.; Mølhave, L.; Massling, A.; Jensen, B.; Grønborg, T.K.; Bossi, R.; Forchhammer, L.; Kjærgaard, S.K.; Sigsgaard, T. Wood smoke in a controlled exposure experiment with human volunteers. Inhal. Toxicol. 2011, 23, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Sehlstedt, M.; Dove, R.; Boman, C.; Pagels, J.; Swietlicki, E.; Löndahl, J.; Swietlicki, E.; Löndahl, J.; Westerholm, R.; Bosson, J.; et al. Antioxidant airway responses following experimental exposure to wood smoke in man. Part. Fibre Toxicol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Duclos, P.; Sanderson, L.M.; Lipsett, M. The 1987 forest fire disaster in California: Assessment of emergency room visits. Arch. Environ. Health 1990, 45, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Slater, D.; Larson, T. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Amer. Rev. Respir. Dis. 1993, 147, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.; YoungPong, S.N.; Koenig, J.Q.; Larson, T.V.; Sheppard, L.; Stout, J.W. An association between fine particles and asthma emergency department visits for children in Seattle. Environ. Health Perspect. 1999, 107, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, A.B.; Larson, T.V.; Sheppard, L.; Claiborn, C.S. Ambient wood smoke and associated respiratory emergency department visits in Spokane, Washington. Int. J. Occup. Environ. Health 2006, 12, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, L.; Levy, D.; Norris, G.; Larson, T.V.; Koenig, J.Q. Effects of ambient air pollution on nonelderly asthma hospital admissions in Seattle, Washington, 1987–1994. Epidemiology 1999, 10, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.A.; Mannino, D.M.; Alverson, C.J.; Kiyu, A.; Hashim, J.; Lee, T.; Falter, K.; Redd, S.C. Cardiorespiratory hospitalizations associated with smoke exposure during the 1997, Southeast Asian forest fires. Int. J. Hyg. Environ. Health 2005, 208, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Wahlin, P.; Raaschou-Nielsen, O.; Scheike, T.; Loft, S. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Brummel, S.; Wu, J.; Stern, H.; Ostro, B.; Lipsett, M.; Winer, A.; Street, D.H.; Zhang, L.; Tjoa, T.; et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup. Environ. Med. 2009, 66, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Sheppeard, V.; Khalaj, B.; Ayyar, A.; Lincoln, D.; Jalaludin, B.; Beard, J.; Corbett, S.; Lumley, T. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 2010, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.B.; Brauer, M.; MacNab, Y.C.; Kennedy, S.M. Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environ. Health Perspect. 2011, 119, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, S.C. Impact to lung health of haze from forest fires: The Singapore experience. Respirology 2000, 5, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.A.; Meyer, P.; Mannino, D.; Redd, S.C.; Smith, E.M.; Gotway-Crawford, C.; Chase, E. Wildland forest fire smoke: Health effects and intervention evaluation, Hoopa, California, 1999. West J. Med. 2002, 176, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Copes, R.; Fisk, R.; Joy, R.; Chan, K.; Brauer, M. Population health effects of air quality changes due to forest fires in British Columbia in 2003: Estimates from physician-visit billing data. Can. J. Public Health 2006, 97, 105–108. [Google Scholar] [PubMed]
- Yu, O.; Sheppard, L.; Lumley, T.; Koenig, J.Q.; Shapiro, G.G. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ. Health Perspect. 2000, 108, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.C.; Lumley, T.; Sheppard, L.; Koenig, J.Q.; Shapiro, G.G. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann. Allergy Asthma Immunol. 2003, 91, 346–353. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Make, B.J.; Vedal, S.; Zhang, L.; Dutton, S.J.; Murphy, J.R.; Silkoff, P.E. Wildfire smoke and respiratory symptoms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2005, 115, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Künzli, N.; Avol, E.; Wu, J.; Gauderman, W.J.; Rappaport, E.; Millstein, J.; Bennion, J.; McConnell, R.; Gilliland, F.D.; Berhane, K.; et al. Health effects of the 2003 Southern California wildfires on children. Amer. J. Respir. Crit. Care Med. 2006, 174, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.Q.; Larson, T.V.; Hanley, Q.S.; Rebolledo, V.; Dumler, K.; Checkoway, H.; Wang, S.Z.; Lin, D.; Pierson, W.E. Pulmonary function changes in children associated with fine particulate matter. Environ. Res. 1993, 63, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.Q.; Jansen, K.; Mar, T.F.; Lumley, T.; Kaufman, J.; Trenga, C.A.; Sullivan, J.; Liu, L.J.; Shapiro, G.G.; Larson, T.V. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ. Health Perspect. 2003, 111, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Trenga, C.A.; Sullivan, J.H.; Schildcrout, J.S.; Shepherd, K.P.; Shapiro, G.G.; Liu, L.J.; Kaufman, J.D.; Koenig, J.Q. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest 2006, 129, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.W.; Mar, T.; Koenig, J.; Liu, L.J.; Gould, T.; Simpson, C.; Larson, T. Changes in lung function and airway inflammation among asthmatic children residing in a wood smoke-impacted urban area. Inhal. Toxicol. 2008, 20, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.C.; Qiu, D.; Liam, B.L.; Ng, T.P.; Lee, S.H.; van Eeden, S.F.; D’Yachkova, Y.; Hogg, J.C. The human bone marrow response to acute air pollution caused by forest fires. Amer. J. Respir. Crit. Care Med. 2000, 161, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.W.; Carlsten, C.; Karlen, B.; Leckie, S.; van Eeden, S.; Vedal, S.; Wong, I.; Brauer, M. An air filter intervention study of endothelial function among healthy adults in a wood smoke-impacted community. Amer. J. Respir. Crit. Care Med. 2011, 183, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, J.A.; Marmur, A.; Klein, M.; Kim, E.; Russell, A.G.; Sarnat, S.E.; Mulholland, J.A.; Hopke, P.K.; Tolbert, P.E. Fine particle sources and cardiorespiratory morbidity: An application of chemical mass balance and factor analytical source-apportionment methods. Environ. Health Perspect. 2008, 116, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Mar, T.F.; Norris, G.A.; Koenig, J.Q.; Larson, T.V. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ. Health Perspect. 2000, 108, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sastry, N. Forest fires, air pollution, and mortality in Southeast Asia. Demography 2002, 39, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Sheppard, L.; Checkoway, H.; Kaufman, J.; Lumley, T.; Koenig, J.; Siscovick, D. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology 2001, 12, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.; Ishikawa, N.; Sheppard, L.; Siscovick, D.; Checkoway, H.; Kaufman, J. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Amer. J. Epidemiol. 2003, 157, 501–519. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Schreuder, A.B.; Trenga, C.A.; Liu, S.L.; Larson, T.V.; Koenig, J.Q.; Kaufman, J.D. Association between short term exposure to fine particulate matter and heart rate variability in older subjects with and without heart disease. Thorax 2005, 60, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Christensen, W.F.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Larson, T.V.; Neas, L.; Hopke, P.K.; et al. PM source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J. Expo. Anal. Environ. Epidemiol. 2005, 16, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Bailie, R.S.; Pilotto, L.S.; Hanigan, I.C. Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. BMC Public Health 2007. [Google Scholar] [CrossRef] [PubMed]
- Vedal, S.; Dutton, S.J. Wildfire air pollution and daily mortality in a large urban area. Environ. Res. 2006, 102, 29–35. [Google Scholar] [CrossRef] [PubMed]
- IEA Bioenergy Biobank Database. 2015. Available online: http://www.ieabcc.nl/database/biobank.html (accessed on 31 May 2011).
- Comparison of Coal Combustion Products to Other Common Materials: Chemical Characteristics; Electric Power Research Institute (EPRI): Palo Alto, CA, USA.
- Hazard Review: Health Effects of Occupational Exposure to Respirable Crystalline Silica; National Institutes of Occupational Safety and Health (NIOSH): Cincinnati, OH, USA.
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima nuclear accidents: A review of the environmental impacts. Sci. Total Environ. 2014, 40–41, 800–817. [Google Scholar] [CrossRef] [PubMed]
- Organo, C.; Lee, E.M.; Menezes, G.; Finch, E.C. Investigation of occupational radiation exposures to NORM at an Irish peat-fired power station and potential use of peat fly ash by the construction industry. J. Radilo. Prot. 2005, 25, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M. Global Wood Pellet Industry Market and Trade Study, IEA Bioenergy Task 40: Sustainable International Bioenergy Trade. 2011. Available online: http://www.bioenergytrade.org/downloads/t40-global-wood-pellet-market-study_final.pdf (accessed on 13 May 2011).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohr, A.C.; Campleman, S.L.; Long, C.M.; Peterson, M.K.; Weatherstone, S.; Quick, W.; Lewis, A. Potential Occupational Exposures and Health Risks Associated with Biomass-Based Power Generation. Int. J. Environ. Res. Public Health 2015, 12, 8542-8605. https://doi.org/10.3390/ijerph120708542
Rohr AC, Campleman SL, Long CM, Peterson MK, Weatherstone S, Quick W, Lewis A. Potential Occupational Exposures and Health Risks Associated with Biomass-Based Power Generation. International Journal of Environmental Research and Public Health. 2015; 12(7):8542-8605. https://doi.org/10.3390/ijerph120708542
Chicago/Turabian StyleRohr, Annette C., Sharan L. Campleman, Christopher M. Long, Michael K. Peterson, Susan Weatherstone, Will Quick, and Ari Lewis. 2015. "Potential Occupational Exposures and Health Risks Associated with Biomass-Based Power Generation" International Journal of Environmental Research and Public Health 12, no. 7: 8542-8605. https://doi.org/10.3390/ijerph120708542
APA StyleRohr, A. C., Campleman, S. L., Long, C. M., Peterson, M. K., Weatherstone, S., Quick, W., & Lewis, A. (2015). Potential Occupational Exposures and Health Risks Associated with Biomass-Based Power Generation. International Journal of Environmental Research and Public Health, 12(7), 8542-8605. https://doi.org/10.3390/ijerph120708542





