Susceptibility to Heat-Related Fluid and Electrolyte Imbalance Emergency Department Visits in Atlanta, Georgia, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Emergency Department Visit Data
2.2. Meteorological Data
2.3. Epidemiologic Analysis
3. Results
3.1. Data Summary
3.2. Associations of FEI ED Visits and Warm-Season Temperature
3.3. Heterogeneity of Heat Morbidity Associations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meehl, G.A.; Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.; Hess, J.J.; Frumkin, H. Urban form and extreme heat events: Are sprawling cities more vulnerable to climate change than compact cities? Environ. Health Perspect. 2010, 118, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Confalonieri, U.; Menne, B.; Akhtar, R.; Ebi, K.L.; Hauengue, M.; Kovats, R.S.; Revich, B.; Woodward, A. Human health. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 391–431. [Google Scholar]
- U.S. Global Change Research Program. Climate Change Impacts in the United States: The Third National Climate Assessment; Melillo, J.M., Richmond, T.C., Yohe, G.W., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2014; p. 841. [Google Scholar]
- Basu, R. High ambient temperature and mortality: A review of epidemiologic studies from 2001 to 2008. Environ. Health 2009. [Google Scholar] [CrossRef] [PubMed]
- Curriero, F.C.; Heiner, K.S.; Samet, J.M.; Zeger, S.L.; Strug, L.; Patz, J.A. Temperature and mortality in 11 cities of the eastern United States. Am. J. Epidemiol. 2002, 155, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramón, M.; Zanobetti, A.; Cavanagh, D.P.; Schwartz, J. Extreme temperatures and mortality: Assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ. Health Perspect. 2006, 114, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.S. Modifiers of the temperature and mortality association in seven US cities. Am. J. Epidemiol. 2003, 157, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, K.; Rotkin-Ellman, M.; King, G.; Margolis, H.G.; Smith, D.; Solomon, G.; Trent, R.; English, P. The 2006 California heat wave: Impacts on hospitalizations and emergency department visits. Environ. Health Perspect. 2009, 117, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Pearson, D.; Malig, B.; Broadwin, R.; Green, R. The effect of high ambient temperature on emergency room visits. Epidemiology 2012, 23, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Green, R.S.; Basu, R.; Malig, B.; Broadwin, R.; Kim, J.J.; Ostro, B. The effect of temperature on hospital admissions in nine California counties. Int. J. Public Health 2010, 55, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pudpong, N.; Hajat, S. High temperature effects on out-patient visits and hospital admissions in Chiang Mai, Thailand. Sci. Total Environ. 2011, 409, 5260–5267. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Samet, J.M.; Patz, J.A. Hospital admissions for heart disease: The effects of temperature and humidity. Epidemiology 2004, 15, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; McCullough, J.E.; Flanders, W.D.; McGeehin, M.A.; Lumpkin, J.R. Excess hospital admissions during the july 1995 heat wave in Chicago. Am. J. Prev. Med. 1999, 16, 269–277. [Google Scholar] [CrossRef]
- Turner, L.R.; Barnett, A.G.; Connell, D.; Tong, S. Ambient temperature and cardiorespiratory morbidity: A systematic review and meta-analysis. Epidemiology 2012, 23, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wolff, R.; Yu, W.; Vaneckova, P.; Pan, X.; Tong, S. Ambient temperature and morbidity: A review of epidemiological evidence. Environ. Health Perspect. 2012, 120, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.L.; Bi, P.; Ryan, P.; Nitschke, M.; Pisaniello, D.; Tucker, G. The effect of heat waves on hospital admissions for renal disease in a temperate city of Australia. Int. J. Epidemiol. 2008, 37, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gu, S.; Bi, P.; Yang, J.; Liu, Q. Heat waves and morbidity: Current knowledge and further direction—A comprehensive literature review. Int. J. Environ. Res. Public Health 2015, 12, 5256–5283. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.K.; Noe, R.S.; Murphy, M.W.; Vaidyanathan, A.; Young, R.; Kieszak, S.; Freymann, G.; Smith, W.; Drenzek, C.; Lewis, L.; et al. Heat illness: Predictors of hospital admissions among emergency department visits—Georgia, 2002–2008. J. Community Health 2014, 39, 90–98. [Google Scholar] [CrossRef] [PubMed]
- McGeehin, M.A.; Mirabelli, M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ. Health Perspect. 2001, 109, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Winquist, A.; Grundstein, A.; Chang, H.H.; Hess, J.; Sarnat, S.E. Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ. Res. 2016, 147, 314–323. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, A.M.; Sahota, O.; Lobo, D.N. Acute and chronic effects of hydration status on health. Nutr. Rev. 2015, 73, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Medicode (Firm). ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification, 5th ed.; Medicode: Salt Lake City, UT, USA, 1997. [Google Scholar]
- Weiss-Guillet, E.M.; Takala, J.; Jakob, S.M. Diagnosis and management of electrolyte emergencies. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 623–651. [Google Scholar] [CrossRef]
- Choudhary, E.; Vaidyanathan, A. Heat stress illness hospitalizations—Environmental Public Health Tracking Program, 20 states, 2001–2010. MMWR Surveill. Summ. 2014, 63, 1–10. [Google Scholar] [PubMed]
- Hess, J.J.; Saha, S.; Luber, G. Summertime acute heat illness in U.S. Emergency departments from 2006 through 2010: Analysis of a nationally representative sample. Environ. Health Perspect. 2014, 122, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.C.; Gao, X.; Liu, G.P.; Wang, W.; Shen, J.M.; Xu, M.J.; Shen, J. Heat-related illness in Jinshan District of Shanghai: A retrospective analysis of 70 patients. World J. Emerg. Med. 2014, 5, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Ding, G.; Gu, S.; Bi, P.; Su, B.; Qin, D.; Xu, G.; Liu, Q. The effects of summer temperature and heat waves on heat-related illness in a coastal city of China, 2011–2013. Environ. Res. 2014, 132, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Le Tertre, A.; Schwartz, J.; Gotway, C.A.; Daley, W.R.; Rubin, C.H. The effect of the 1995 heat wave in Chicago on all-cause and cause-specific mortality. Am. J. Public Health 2007, 97, S158–S162. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.S.; Kinney, P.L.; Cohen, A.J. Environmental equity in air quality management: Local and international implications for human health and climate change. J. Toxicol. Environ. Health A 2008, 71, 570–577. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.S.; Zanobetti, A.; Schwartz, J. Disparities by race in heat-related mortality in four US cities: The role of air conditioning prevalence. J. Urban Health 2005, 82, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kinney, P.L.; O’Neill, M.S.; Bell, M.L.; Schwartz, J. Approaches for estimating effects of climate change on heat-related deaths: Challenges and opportunities. Environ. Sci. Policy 2008, 11, 87–96. [Google Scholar] [CrossRef]
- Dei Cas, L.; Metra, M.; Leier, C.V. Electrolyte disturbances in chronic heart failure: Metabolic and clinical aspects. Clin. Cardiol. 1995, 18, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Milionis, H.J.; Alexandrides, G.E.; Liberopoulos, E.N.; Bairaktari, E.T.; Goudevenos, J.; Elisaf, M.S. Hypomagnesemia and concurrent acid-base and electrolyte abnormalities in patients with congestive heart failure. Eur. J. Heart Fail. 2002, 4, 167–173. [Google Scholar] [CrossRef]
- Goldberg, A.; Hammerman, H.; Petcherski, S.; Nassar, M.; Zdorovyak, A.; Yalonetsky, S.; Kapeliovich, M.; Agmon, Y.; Beyar, R.; Markiewicz, W.; et al. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch. Intern. Med. 2006, 166, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Hammerman, H.; Petcherski, S.; Zdorovyak, A.; Yalonetsky, S.; Kapeliovich, M.; Agmon, Y.; Markiewicz, W.; Aronson, D. Prognostic importance of hyponatremia in acute st-elevation myocardial infarction. Am. J. Med. 2004, 117, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.P.; Lobo, D.N. Fluid and electrolytes in the elderly. Curr. Opin. Clin. Nutr Metab. Care 2004, 7, 23–33. [Google Scholar] [CrossRef]
- Ito, H.; Fujimaki, H.; Inoue, J.; Shiraki, M. Disorders of fluid and electrolyte metabolism in elderly diabetics. Nihon Ronen Igakkai Zasshi 1989, 26, 233–239. [Google Scholar] [PubMed]
- Schwartz, J. Who is sensitive to extremes of temperature?: A case-only analysis. Epidemiology 2005, 16, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Hess, J.; Rogers, C.A.; Tolbert, P.E.; Klein, M.; Sarnat, S.E. Ambient pollen concentrations and emergency department visits for asthma and wheeze. J. Allergy Clin. Immunol. 2012, 130, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.B.; Tolbert, P.E.; Klein, M.; Peel, J.L.; Flanders, W.D.; Todd, K.; Mulholland, J.A.; Ryan, P.B.; Frumkin, H. Ambient air pollution and cardiovascular emergency department visits. Epidemiology 2004, 15, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Peel, J.L.; Tolbert, P.E.; Klein, M.; Metzger, K.B.; Flanders, W.D.; Todd, K.; Mulholland, J.A.; Ryan, P.B.; Frumkin, H. Ambient air pollution and respiratory emergency department visits. Epidemiology 2005, 16, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, S.E.; Klein, M.; Sarnat, J.A.; Mulholland, J.; Russell, A.G.; Flanders, W.D.; Waller, L.A.; Tolbert, P.E. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.J.; Darrow, L.A.; Klein, M.; Flanders, W.D.; Sarnat, J.A.; Waller, L.A.; Sarnat, S.E.; Mulholland, J.A.; Tolbert, P.E. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am. J. Respir. Crit. Care Med. 2010, 182, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, P.E.; Klein, M.; Metzger, K.B.; Peel, J.; Flanders, W.D.; Todd, K.; Mulholland, J.A.; Ryan, P.B.; Frumkin, H. Interim results of the study of particulates and health in Atlanta (SOPHIA). J. Expo. Anal. Environ. Epidemiol. 2000, 10, 446–460. [Google Scholar] [CrossRef] [PubMed]
- GeoLytics, Inc. The Time-Series Research Package; GeoLytics, Inc.: East Brunswick, NJ, USA, 2013. [Google Scholar]
- O’Lenick, C.; Winquist, A.; Mulholland, J.A.; Friberg, M.; Chang, H.H.; Kramer, M.; Darrow, L.A.; Sarnat, S.E. Assessment of neighborhood-level socioeconomic status as a modifier of air pollution-asthma associations among children in Atlanta. J. Epidemiol. Community Health 2016. [Google Scholar] [CrossRef] [PubMed]
- Steadman, R.G. A universal scale of apparent temperature. J. Clim. Appl. Meteorol. 1984, 23, 1674–1687. [Google Scholar] [CrossRef]
- Anderson, G.B.; Bell, M.L.; Peng, R.D. Methods to calculate the heat index as an exposure metric in environmental health research. Environ. Health Perspect. 2013, 121, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.S.; MacLehose, R.F. Which of these things is not like the others? Cancer 2013, 119, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Astrom, D.O.; Forsberg, B.; Rocklov, J. Heat wave impact on morbidity and mortality in the elderly population: A review of recent studies. Maturitas 2011, 69, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Borrell, C.; Mari-Dell’Olmo, M.; Rodriguez-Sanz, M.; Garcia-Olalla, P.; Cayla, J.A.; Benach, J.; Muntaner, C. Socioeconomic position and excess mortality during the heat wave of 2003 in Barcelona. Eur. J. Epidemiol. 2006, 21, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, A.; Rey, G.; Laurent, F.; Pavillon, G.; Bellec, S.; Guihenneuc-Jouyaux, C.; Clavel, J.; Jougla, E.; Hemon, D. Excess mortality related to the august 2003 heat wave in France. Int. Arch. Occup. Environ. Health 2006, 80, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajat, S.; Kovats, R.S.; Lachowycz, K. Heat-related and cold-related deaths in England and Wales: Who is at risk? Occup. Environ. Med. 2007, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, A.; Hajat, S.; Kovats, R.S.; Bisanti, L.; Rognoni, M.; Russo, A.; Paldy, A. An ecological time-series study of heat-related mortality in three European cities. Environ. Health 2008. [Google Scholar] [CrossRef] [PubMed]
- Kysely, J. Mortality and displaced mortality during heat waves in the Czech Republic. Int. J. Biometeorol. 2004, 49, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Martiello, M.A.; Giacchi, M.V. High temperatures and health outcomes: A review of the literature. Scand. J. Public Health 2010, 38, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Michelozzi, P.; de’Donato, F.; Accetta, G.; Forastiere, F.; D’Ovidio, M.; Perucci, C.; Kalkstein, L. Impact of heat waves on mortality—Rome, Italy, June–August 2003. JAMA 2004, 291, 2537–2538. [Google Scholar]
- Rainham, D.G.C.; Smoyer-Tomic, K.E. The role of air pollution in the relationship between a heat stress index and human mortality in Toronto. Environ. Res. 2003, 93, 9–19. [Google Scholar] [CrossRef]
- Rey, G.; Jougla, E.; Fouillet, A.; Pavillon, G.; Bessemoulin, P.; Frayssinet, P.; Clavel, J.; Hemon, D. The impact of major heat waves on all-cause and cause-specific mortality in France from 1971 to 2003. Int. Arch. Occup. Environ. Health 2007, 80, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Rooney, C.; McMichael, A.J.; Kovats, R.S.; Coleman, M.P. Excess mortality in England and Wales, and in Greater London, during the 1995 heatwave. J. Epidemiol. Community Health 1998, 52, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Forastiere, F.; Agostini, D.; Biggeri, A.; Bisanti, L.; Cadum, E.; Caranci, N.; de’Donato, F.; De Lisio, S.; De Maria, M.; et al. Vulnerability to heat-related mortality—A multicity, population-based, case-crossover analysis. Epidemiology 2006, 17, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Beggs, P.J.; Vaneckova, P. Admission to hospital for effects of heat and light: NSW, 1993-94 to 2003-04. N S. W. Public Health Bull. 2008, 19, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Schmeltz, M.T.; Sembajwe, G.; Marcotullio, P.J.; Grassman, J.A.; Himmelstein, D.U.; Woolhandler, S. Identifying individual risk factors and documenting the pattern of heat-related illness through analyses of hospitalization and patterns of household cooling. PLoS ONE 2015, 10, e0118958. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.A.; Thomas, K.E.; Malilay, J.; Annest, J.L. Nonfatal natural and environmental injuries treated in emergency departments, United States, 2001–2004. Fam. Community Health 2010, 33, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Uejio, C.; Wilhelmi, O.; Golden, J.; Mills, D.; Gulino, S.; Samenow, J. Intra-urban societal vulnerability to extreme heat: The role of heat exposure and the built environment, socioeconomics, and neighborhood stability. Health Place 2011, 17, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, C.J. Racial and socioeconomic disparities in heat-related health effects and their mechanisms: A review. Curr. Epidemiol. Rep. 2014, 1, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Harlan, S.L.; Brazel, A.J.; Prashad, L.; Stefanov, W.L.; Larsen, L. Neighborhood microclimates and vulnerability to heat stress. Soc. Sci. Med. 2006, 63, 2847–2863. [Google Scholar] [CrossRef] [PubMed]
- Harlan, S.L.; Declet-Barreto, J.H.; Stefanov, W.L.; Petitti, D.B. Neighborhood effects on heat deaths: Social and environmental predictors of vulnerability in Maricopa County, Arizona. Environ. Health Perspect. 2013, 121, 197–204. [Google Scholar] [PubMed]
- Stoecklin-Marois, M.; Hennessy-Burt, T.; Mitchell, D.; Schenker, M. Heat-related illness knowledge and practices among California hired farm workers in the MICASA study. Ind. Health 2013, 51, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Michelozzi, P.; de Donato, F.; Bisanti, L.; Russo, A.; Cadum, E.; DeMaria, M.; D’Ovidio, M.; Costa, G.; Perucci, C.A. The impact of the summer 2003 heat waves on mortality in four Italian cities. Euro. Surveill. 2005, 10, 161–165. [Google Scholar] [PubMed]
- Basu, R.; Ostro, B.D. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am. J. Epidemiol. 2008, 168, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Carcaillon, L.; Lefranc, A.; Helmer, C.; Dartigues, J.F.; Tavernier, B.; Ledrans, M.; Filleul, L. Factors associated with morbidity during the 2003 heat wave in two population-based cohorts of elderly subjects: PAQUID and three city. Eur. J. Epidemiol. 2008, 23, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, C.M.; Sugg, M.M.; Konrad, C.E., 2nd; Waller, A. Impact of extreme heat events on emergency department visits in North Carolina (2007–2011). J. Community Health 2016, 41, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Hajat, S.; Wilkinson, P. Contrasting patterns of mortality and hospital admissions during hot weather and heat waves in Greater London, UK. Occup. Environ. Med. 2004, 61, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.; Diaz, J. Impact of high temperatures on hospital admissions: Comparative analysis with previous studies about mortality (Madrid). Eur. J. Public Health 2008, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Mahaldar, A.R. Acid base and fluid electrolyte disturbances in chronic kidney disease. Clin. Queries: Nephrol. 2012, 1, 295–299. [Google Scholar] [CrossRef]
- Collins, K.J. Fluid balance of elderly people in hot environments. Geriatr. Med. 2009, 39, 385–391. [Google Scholar]
- Khalaj, B.; Lloyd, G.; Sheppeard, V.; Dear, K. The health impacts of heat waves in five regions of New South Wales, Australia: A case-only analysis. Int. Arch. Occup. Environ. Health 2010, 83, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Kovats, R.S.; Atkinson, R.W.; Haines, A. Impact of hot temperatures on death in London: A time series approach. J. Epidemiol. Community Health 2002, 56, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.M.; Liu, W.P.; Chou, S.Y.; Kuo, H.W. Ambient temperature and emergency room admissions for acute coronary syndrome in Taiwan. Int. J. Biometeor 2008, 52, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.M.; Liu, W.P.; Kuo, H.W. Diurnal temperature range and emergency room admissions for chronic obstructive pulmonary disease in Taiwan. Int. J. Biometeorol. 2009, 53, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Fedeli, U.; Visentin, C.; Milan, G.; Fadda, E.; Spolaore, P. Pattern and determinants of hospitalization during heat waves: An ecologic study. BMC Public Health 2007. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.B.; Ito, K.; Matte, T.D. Summer heat and mortality in New York City: How hot is too hot? Environ. Health Perspect. 2010, 118, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Sheridan, S.C.; Allen, M.J.; Pascal, M.; Laaidi, K.; Yagouti, A.; Bickis, U.; Tobias, A.; Bourque, D.; Armstrong, B.G.; et al. Heat–health warning systems: A comparison of the predictive capacity of different approaches to identifying dangerously hot days. Am. J. Public Health 2010, 100, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Morabito, M.; Crisci, A.; Messeri, A.; Capecchi, V.; Modesti, P.A.; Gensini, G.F.; Orlandini, S. Environmental temperature and thermal indices: What is the most effective predictor of heat-related mortality in different geographical contexts? Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Y.; Schwartz, J.D.; O’Neill, M.S. What weather variables are important in predicting heat-related mortality? A new application of statistical learning methods. Environ. Res. 2014, 132, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Rood, R.B.; Michailidis, G.; Oswald, E.M.; Schwartz, J.D.; Zanobetti, A.; Ebi, K.L.; O’Neill, M.S. Comparing exposure metrics for classifying ‘dangerous heat’ in heat wave and health warning systems. Environ. Int. 2012, 46, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Tong, S.; Clements, A.C.A. What measure of temperature is the best predictor of mortality? Environ. Res. 2010, 110, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petitti, D.B.; Hondula, D.M.; Yang, S.; Harlan, S.L.; Chowell, G. Multiple trigger points for quantifying heat-health impacts: New evidence from a hot climate. Environ. Health Perspect. 2016, 124, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Hondula, D.M.; Patel, A.P. Temperature observation time and type influence estimates of heat-related mortality in seven US cities. Environ. Health Perspect. 2016, 124, 795–804. [Google Scholar] [PubMed]
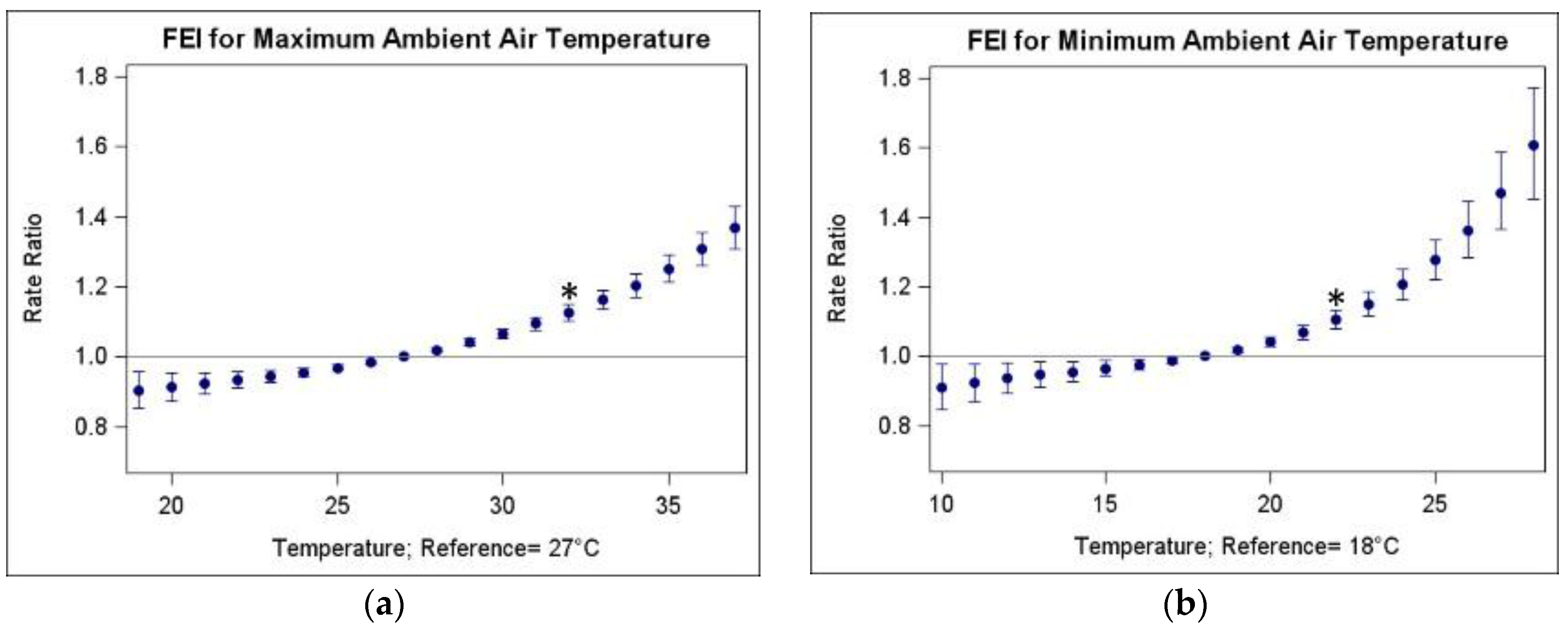
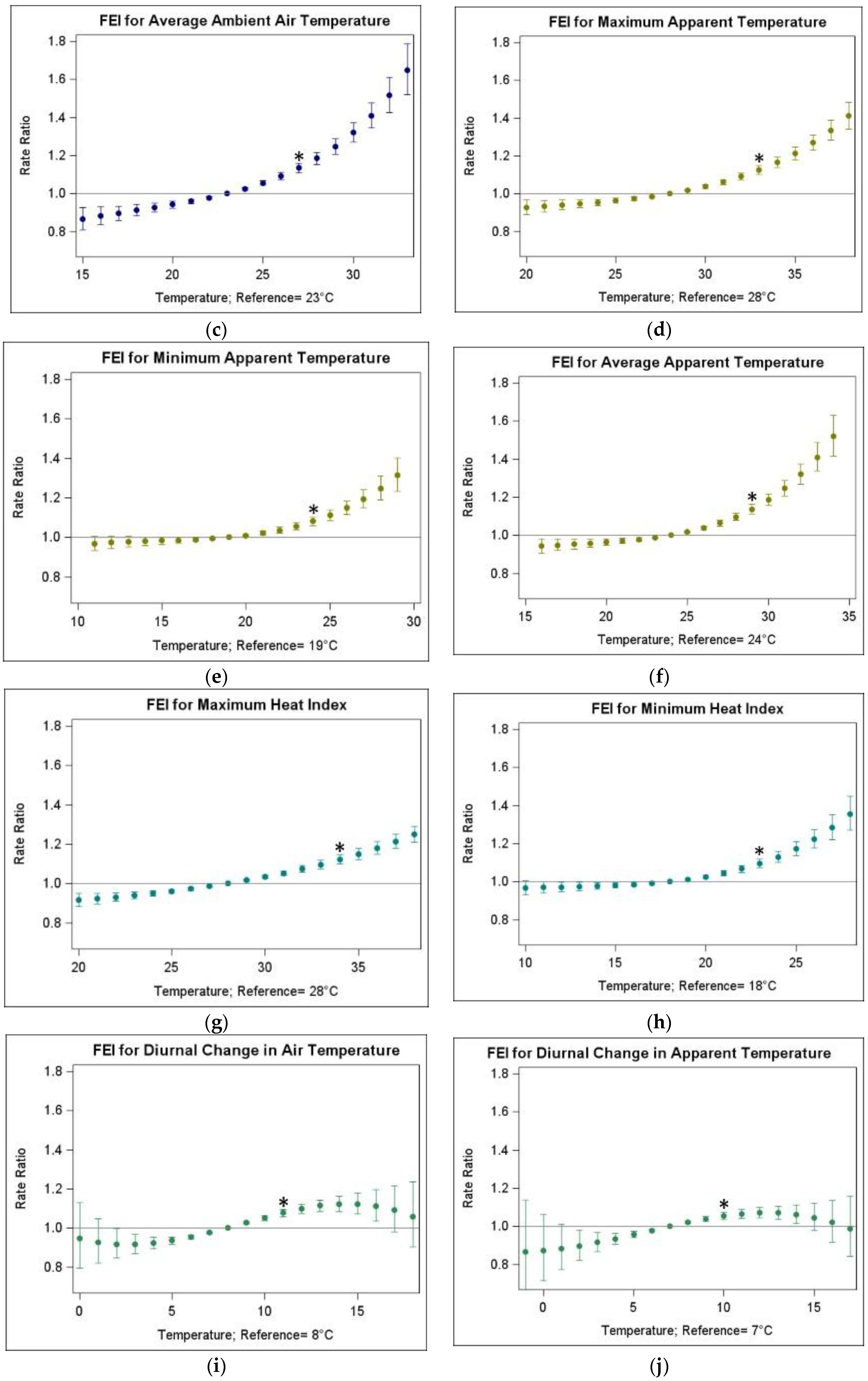
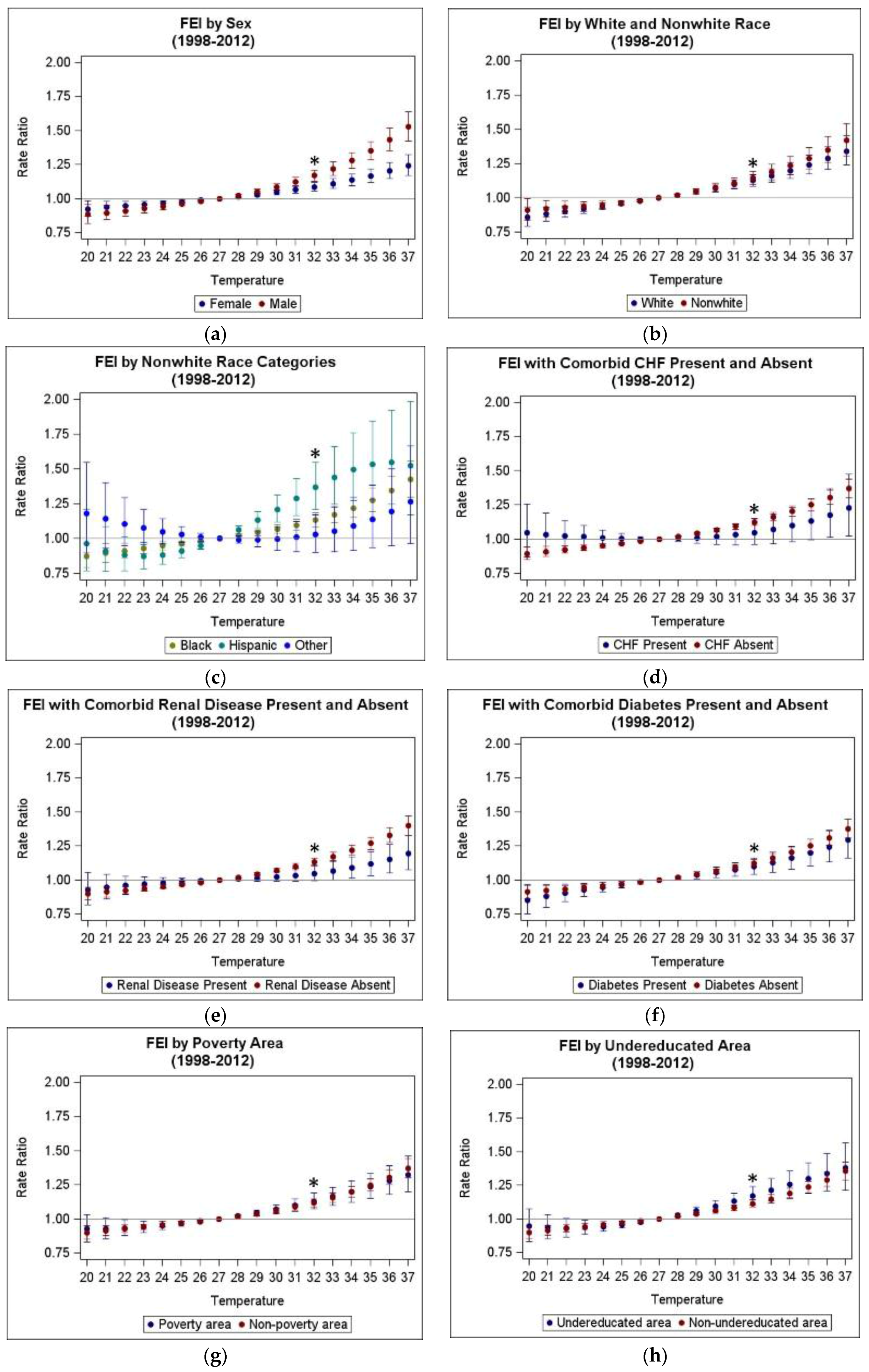
| Temperature Metric | Abbrev. | Mean | SD | Min | 25th Pctl | 50th Pctl | 75th Pctl | Max |
|---|---|---|---|---|---|---|---|---|
| Maximum ambient air temperature | TMax | 29.4 | 3.7 | 13.3 | 27.2 | 30.0 | 32.0 | 40.6 |
| Minimum ambient air temperature | TMin | 19.9 | 3.4 | 5.0 | 18.3 | 20.6 | 22.2 | 28.3 |
| Average ambient air temperature | TAvg | 24.7 | 3.4 | 11.7 | 22.8 | 25.3 | 27.0 | 33.9 |
| Maximum apparent temperature | ATMax | 30.3 | 3.9 | 14.2 | 28.0 | 30.8 | 33.0 | 40.2 |
| Minimum apparent temperature | ATMin | 21.4 | 4.1 | 5.1 | 19.3 | 22.6 | 24.4 | 30.4 |
| Average apparent temperature | ATAvg | 25.9 | 3.8 | 11.3 | 23.8 | 26.7 | 28.6 | 34.9 |
| Maximum heat index | HIMax | 30.6 | 4.6 | 13.2 | 27.8 | 30.8 | 33.8 | 43.4 |
| Minimum heat index | HIMin | 20.2 | 3.8 | 4.0 | 18.2 | 21.2 | 22.9 | 30.8 |
| Diurnal change in air temperature | TDC | 9.5 | 2.5 | 1.1 | 7.8 | 9.5 | 11.1 | 17.2 |
| Diurnal change in apparent temperature | ATDC | 8.8 | 2.2 | 1.4 | 7.5 | 8.8 | 10.3 | 17.2 |
| Temperature Metric | TMax | TMin | TAvg | ATMax | ATMin | ATAvg | HIMax | HIMin | TDC | ATDC |
|---|---|---|---|---|---|---|---|---|---|---|
| TMax | 1.00 | |||||||||
| TMin | 0.75 * | 1.00 | ||||||||
| TAvg | 0.95 * | 0.91 * | 1.00 | |||||||
| ATMax | 0.96 * | 0.84 * | 0.97 * | 1.00 | ||||||
| ATMin | 0.71 * | 0.99 * | 0.89 * | 0.82 * | 1.00 | |||||
| ATAvg | 0.88 * | 0.95 * | 0.98 * | 0.96 * | 0.95 * | 1.00 | ||||
| HIMax | 0.94 * | 0.84 * | 0.96 * | 0.99 * | 0.83 * | 0.96 * | 1.00 | |||
| HIMin | 0.73 * | 1.00 * | 0.90 * | 0.83 * | 1.0 * | 0.95 * | 0.84 * | 1.00 | ||
| TDC | 0.43 * | −0.19 * | 0.15 * | 0.26 * | −0.23 * | 0.03 | 0.23 * | −0.21 * | 1.00 | |
| ATDC | 0.34 * | −0.27 * | 0.07 * | 0.22 * | −0.30 * | −0.04 | 0.20 * | −0.29 * | 0.96 * | 1.00 |
| Temperature Metric | Abbreviation | IQR (25th–75th Percentile, in °C) | RR (95% CI) |
|---|---|---|---|
| Maximum ambient air temperature | TMax | 5 (27–32) | 1.125 (1.102, 1.150) |
| Minimum ambient air temperature | TMin | 4 (18–22) | 1.105 (1.077, 1.133) |
| Average ambient air temperature | TAvg | 4 (23–27) | 1.134 (1.109, 1.159) |
| Maximum apparent temperature | ATMax | 5 (28–33) | 1.125 (1.103, 1.149) |
| Minimum apparent temperature | ATMin | 5 (19–24) | 1.080 (1.058, 1.102) |
| Average apparent temperature | ATAvg | 5 (24–29) | 1.137 (1.113, 1.162) |
| Maximum heat index | HIMax | 6 (28–34) | 1.122 (1.098, 1.146) |
| Minimum heat index | HIMin | 5 (18–23) | 1.096 (1.072, 1.120) |
| Diurnal change in air temperature | TDC | 3 (8–11) | 1.077 (1.058, 1.096) |
| Diurnal change in apparent temperature | ATDC | 3 (7–10) | 1.055 (1.036, 1.073) |
| Modifier | Strata | # of Days | Total FEI ED Visits | Total FEI ED Visits with Modifier Information 1 | Mean (SD) of Daily FEI ED Visits |
|---|---|---|---|---|---|
| All FEI ED visits | --- | 3060 | 66,369 | --- | 21.7 (12.8) |
| Sex | Female | 3060 | 38,426 | 65,713 | 12.6 (7.6) |
| Male | 3060 | 27,287 | 8.9 (6.1) | ||
| Race 2 | Black | 2601 | 14,972 | 44,384 | 5.8 (5.3) |
| Hispanic | 2601 | 1938 | 0.7 (1.2) | ||
| Other | 2601 | 1646 | 0.6 (0.9) | ||
| White | 2601 | 25,828 | 9.9 (6.6) | ||
| Comorbid Congestive Heart Failure | Present | 3060 | 3566 | 66,369 | 1.2 (1.4) |
| Absent | 3060 | 62,803 | 20.5 (12.0) | ||
| Comorbid Renal Disease | Present | 3060 | 9477 | 66,369 | 3.1 (3.6) |
| Absent | 3060 | 56,892 | 18.6 (10.1) | ||
| Comorbid Diabetes | Present | 3060 | 9737 | 66,369 | 3.2 (3.1) |
| Absent | 3060 | 56,632 | 18.5 (10.4) | ||
| Poverty Area 3 | Yes | 3060 | 11,623 | 66,343 | 3.8 (3.5) |
| No | 3060 | 54,720 | 17.9 (10.2) | ||
| Undereducated Area 4 | Yes | 3060 | 9005 | 66,343 | 2.9 (2.0) |
| No | 3060 | 57,338 | 18.7 (12.0) |
| Modifier | Strata | TMax RR (95% CI) All Years Included (1993–2012) | TMax RR (95% CI) Reduced Period (1998–2012) | ATMax RR (95% CI) All Years Included (1993–2012) | ATMax RR (95% CI) Reduced Period (1998–2012) |
|---|---|---|---|---|---|
| Overall | 1.125 (1.102, 1.150) ** | 1.121 (1.096 1.1460) ** | 1.125 (1.103, 1.149) ** | 1.123 (1.099, 1.147) ** | |
| Sex | Female | 1.088 (1.060, 1.118) ** | 1.085 (1.054, 1.117) ** | 1.085 (1.057, 1.114) ** | 1.085 (1.055, 1.116) ** |
| Male | 1.176 (1.138, 1.216) **,^ | 1.169 (1.128, 1.210) **,^ | 1.181 (1.143, 1.219) **,^ | 1.174 (1.135, 1.214) **,^ | |
| Race 1 | White | 1.141 (1.102, 1.180) ** | 1.128 (1.087, 1.170) ** | 1.127 (1.090, 1.164) ** | 1.116 (1.077, 1.156) ** |
| Nonwhite | 1.157 (1.113, 1.202) ** | 1.145 (1.099, 1.193) ** | 1.159 (1.116, 1.204) ** | 1.150 (1.105, 1.197) ** | |
| Black | 1.143 (1.095, 1.193) ** | 1.132 (1.081, 1.185) ** | 1.143 (1.097, 1.192) ** | 1.135 (1.086, 1.187) ** | |
| Hispanic | 1.366 (1.211, 1.541) ** | 1.368 (1.209, 1.549) ** | 1.304 (1.159, 1.466) ** | 1.304 (1.155, 1.471) ** | |
| Other | -- | 1.028 (0.902, 1.171) | -- | 1.072 (0.962, 1.195) | |
| Comorbid Congestive Heart Failure | Present | -- | 1.048 (0.959, 1.145) | -- | 1.069 (0.981, 1.166) |
| Absent | 1.130 (1.106, 1.155) ** | 1.125 (1.099, 1.151) ** | 1.129 (1.105, 1.153) ** | 1.126 (1.101, 1.151) ** | |
| Comorbid Renal Disease | Present | 1.048 (0.995, 1.104) | 1.049 (0.993, 1.108) | 1.069 (1.016, 1.125) | 1.071 (1.014, 1.130) * |
| Absent | 1.137 (1.112, 1.164) **,^ | 1.137 (1.112, 1.164) **,^ | 1.134 (1.110, 1.159) **,^ | 1.132 (1.106, 1.159) ** | |
| Comorbid Diabetes | Present | 1.096 (1.040, 1.156) * | 1.100 (1.040, 1.163) * | 1.102 (1.047, 1.160) * | 1.106 (1.047, 1.167) * |
| Absent | 1.130 (1.105, 1.156) ** | 1.124 (1.098, 1.152) ** | 1.129 (1.105, 1.154) ** | 1.126 (1.100, 1.152) ** | |
| Poverty Area | Yes | 1.132 (1.079, 1.187) ** | 1.130 (1.075, 1.188) ** | 1.131 (1.080, 1.185) ** | 1.128 (1.074, 1.183) ** |
| No | 1.125 (1.099, 1.151) ** | 1.119 (1.092, 1.147) ** | 1.125 (1.100, 1.150) ** | 1.122 (1.096, 1.149) ** | |
| Undereducated Area | Yes | 1.175 (1.114, 1.239) ** | 1.172 (1.104, 1.244) ** | 1.183 (1.124, 1.245) ** | 1.179 (1.113, 1.248) ** |
| No | 1.118 (1.093, 1.144) ** | 1.114 (1.087, 1.141) ** | 1.116 (1.092, 1.141) ** | 1.115 (1.089, 1.141) ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, L.; Winquist, A.; Klein, M.; O’Lenick, C.; Grundstein, A.; Ebelt Sarnat, S. Susceptibility to Heat-Related Fluid and Electrolyte Imbalance Emergency Department Visits in Atlanta, Georgia, USA. Int. J. Environ. Res. Public Health 2016, 13, 982. https://doi.org/10.3390/ijerph13100982
Heidari L, Winquist A, Klein M, O’Lenick C, Grundstein A, Ebelt Sarnat S. Susceptibility to Heat-Related Fluid and Electrolyte Imbalance Emergency Department Visits in Atlanta, Georgia, USA. International Journal of Environmental Research and Public Health. 2016; 13(10):982. https://doi.org/10.3390/ijerph13100982
Chicago/Turabian StyleHeidari, Leila, Andrea Winquist, Mitchel Klein, Cassandra O’Lenick, Andrew Grundstein, and Stefanie Ebelt Sarnat. 2016. "Susceptibility to Heat-Related Fluid and Electrolyte Imbalance Emergency Department Visits in Atlanta, Georgia, USA" International Journal of Environmental Research and Public Health 13, no. 10: 982. https://doi.org/10.3390/ijerph13100982






