Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus–Uterus in Pubertal Female Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Tissue Collection
2.3. Quantification of GnRH
2.4. Immunohistochemistry
2.5. RNA Extraction
2.6. Real-Time Reverse Transcription-PCR
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. General Toxicity and Reproductive Toxicity of DEHP in Pubertal Female Rats
- (1)
- The rats treated with DEHP appeared less active and less spirited, moved more slowly, had untidy and lusterless fur and lost hair. These changes were most obvious in the group of 1000 mg/kg/d DEHP.
- (2)
- (3)
- The ovaries of the rats treated with DEHP were congestive and swelling, and the volume of the ovaries and the uterus became bigger (Figure 1).
- (4)
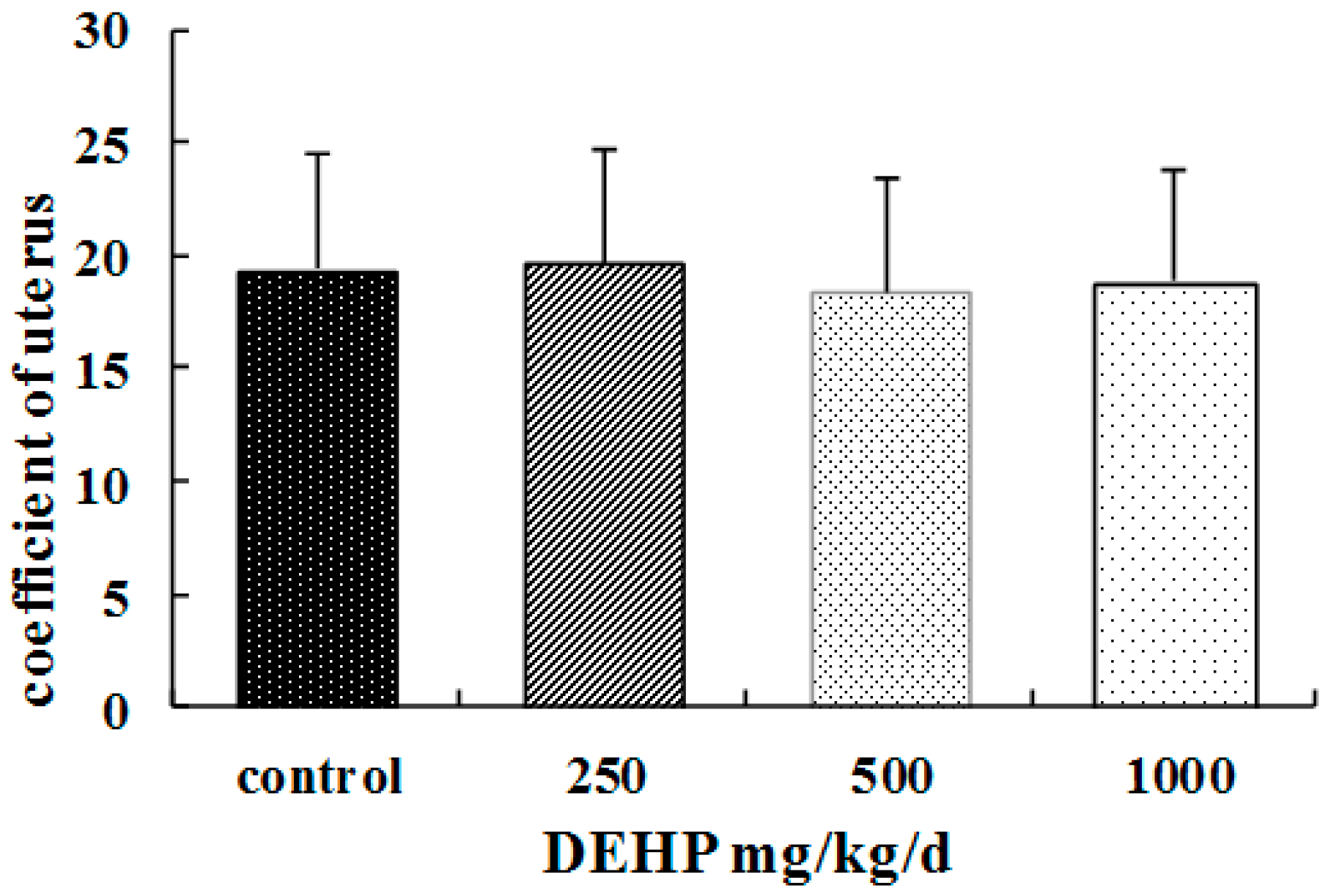
- Compared with the control group, there were no significant differences of the coefficients in uteruses of the rats treated with DEHP (p > 0.05, Figure 2).
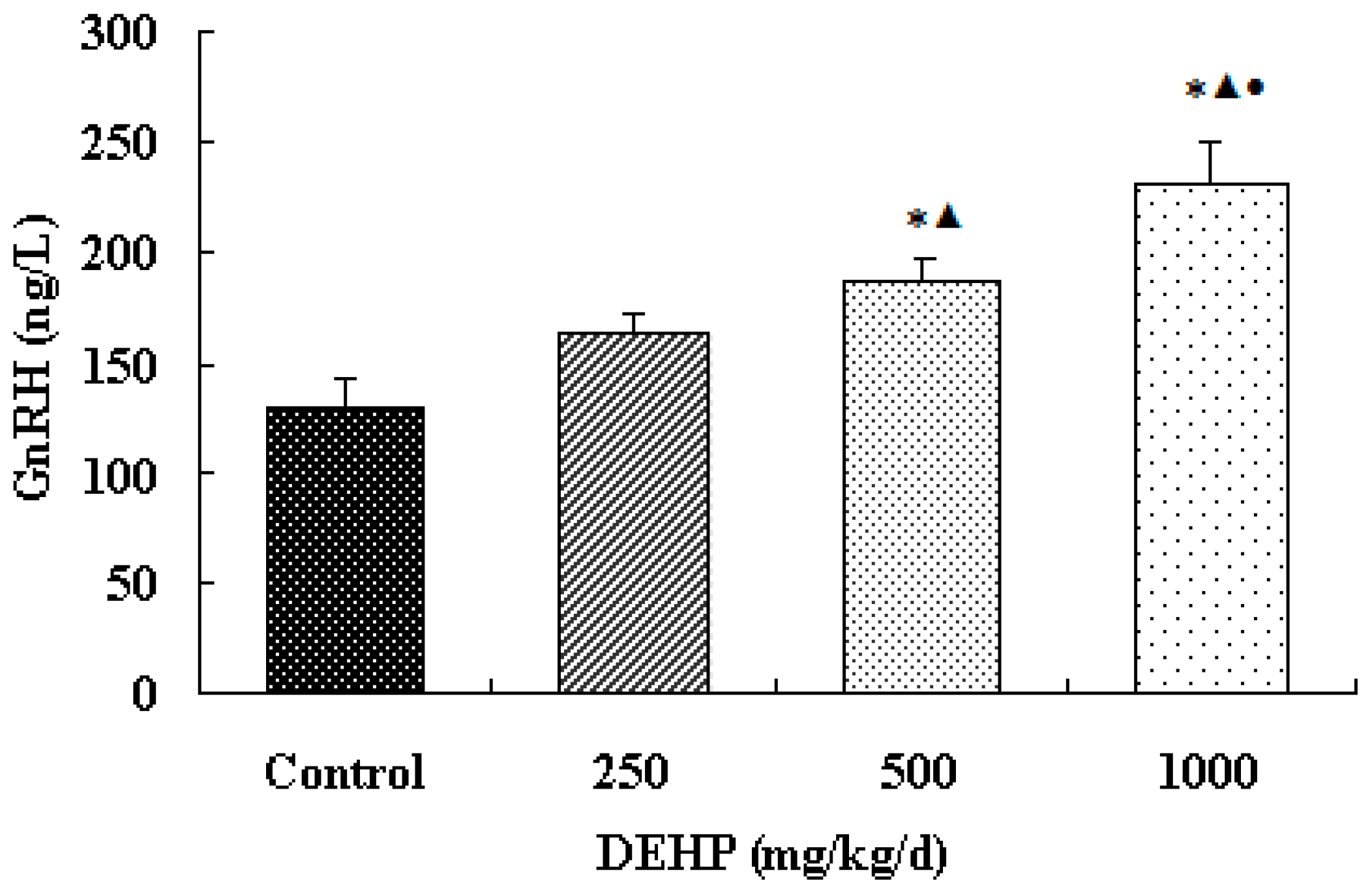
3.2. Effect of DEHP on GnRH Level
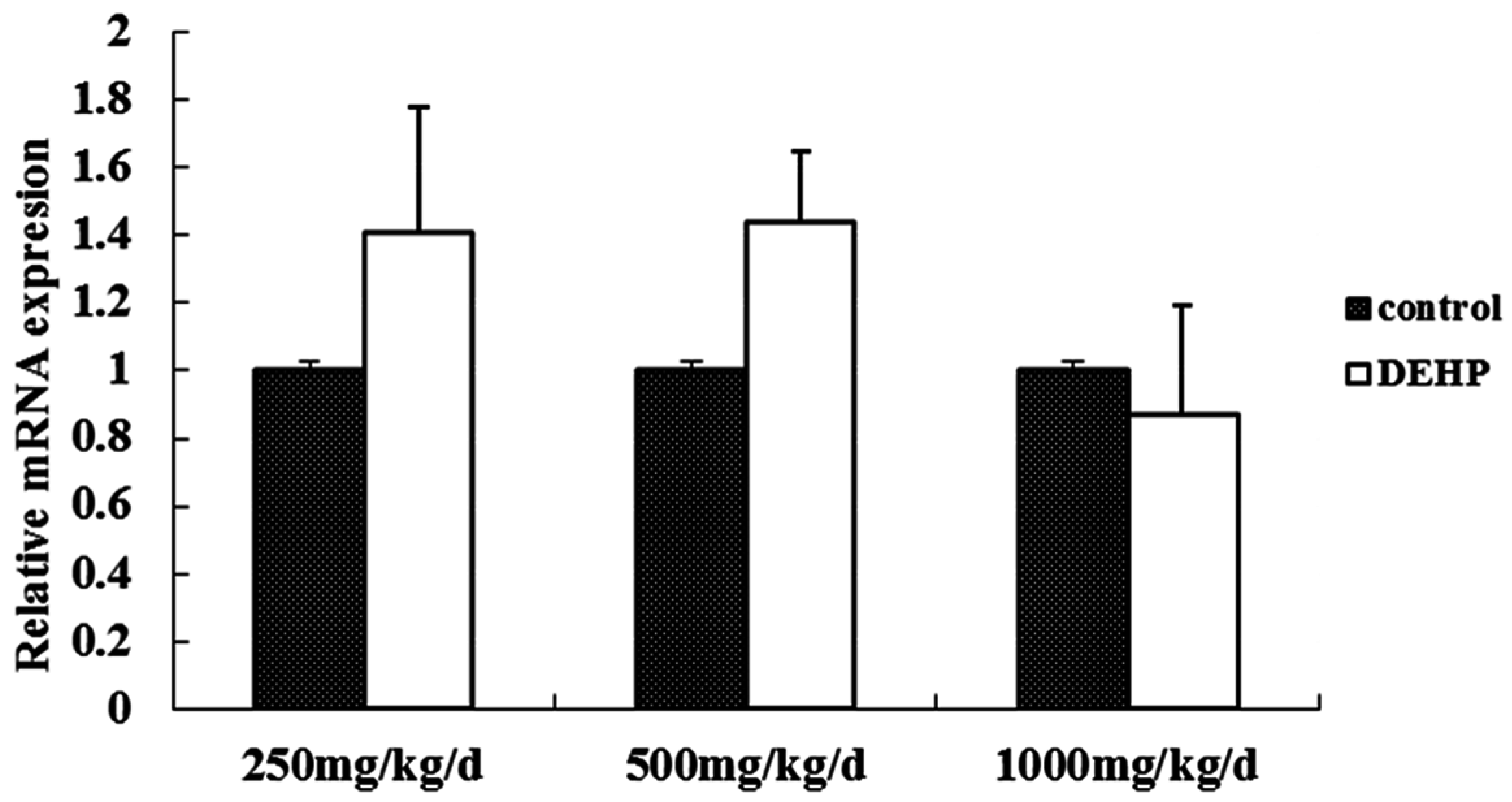
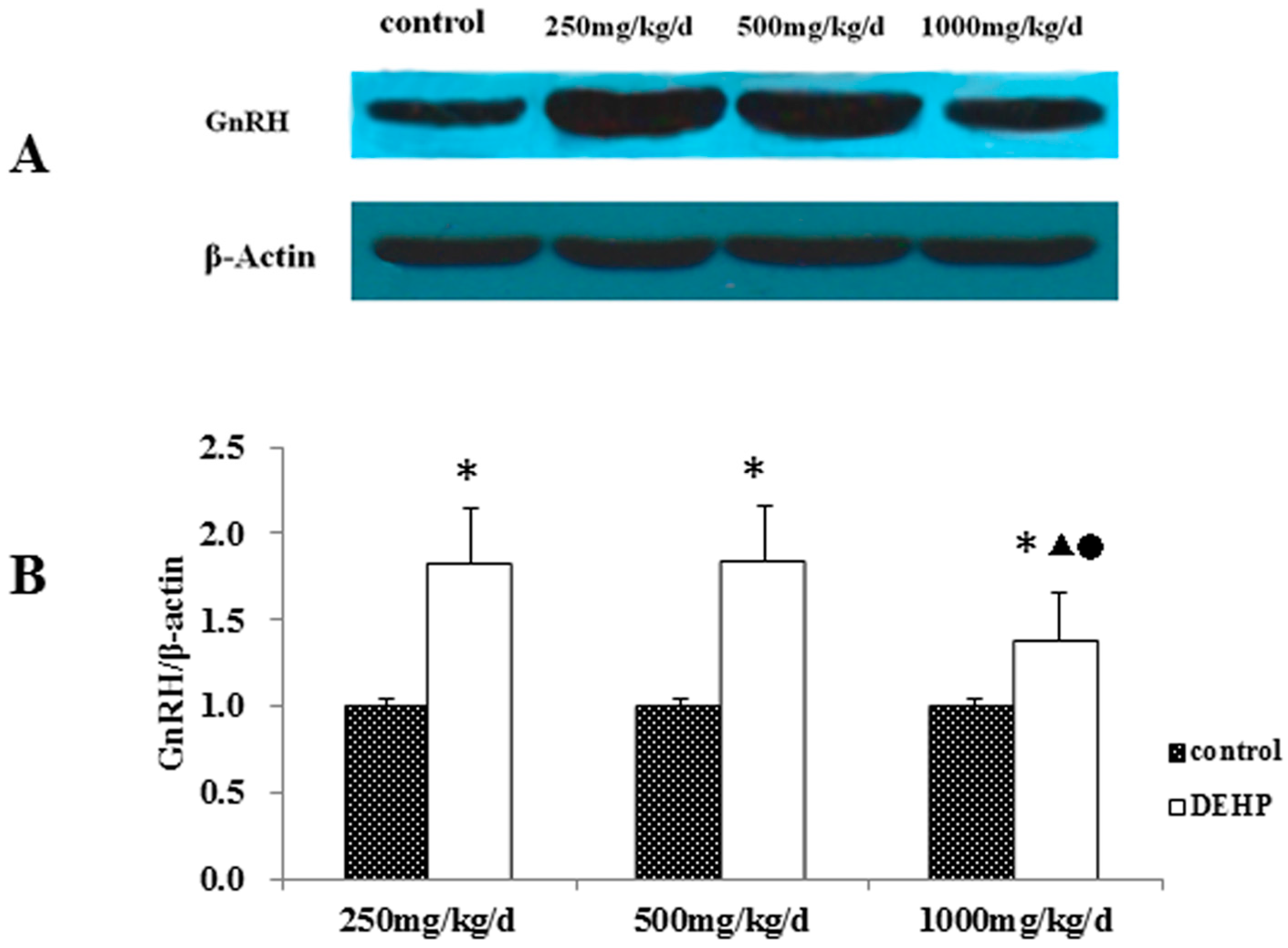
3.3. Gene and Protein Expression Levels of GnRH in the Hypothalamus
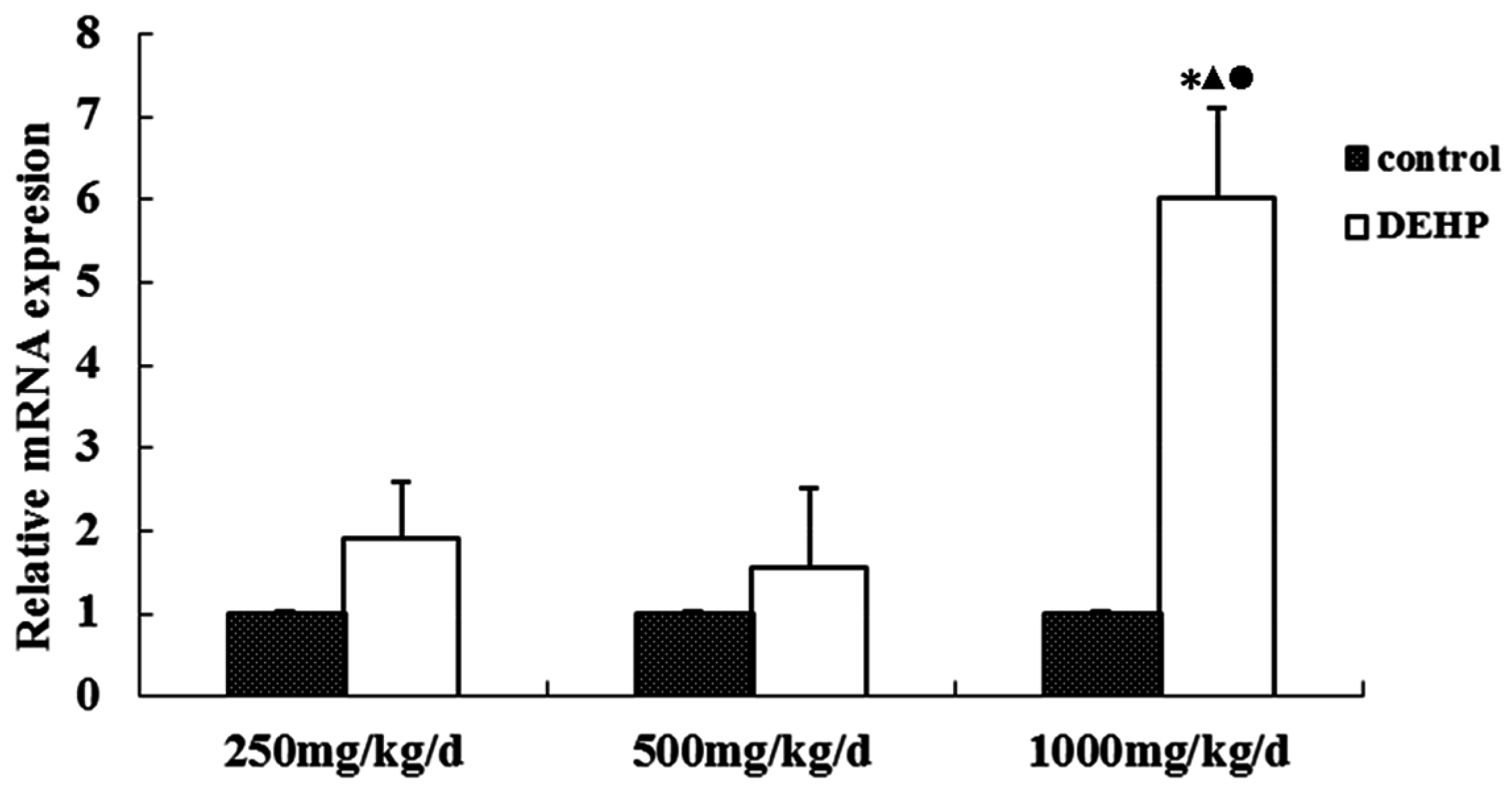
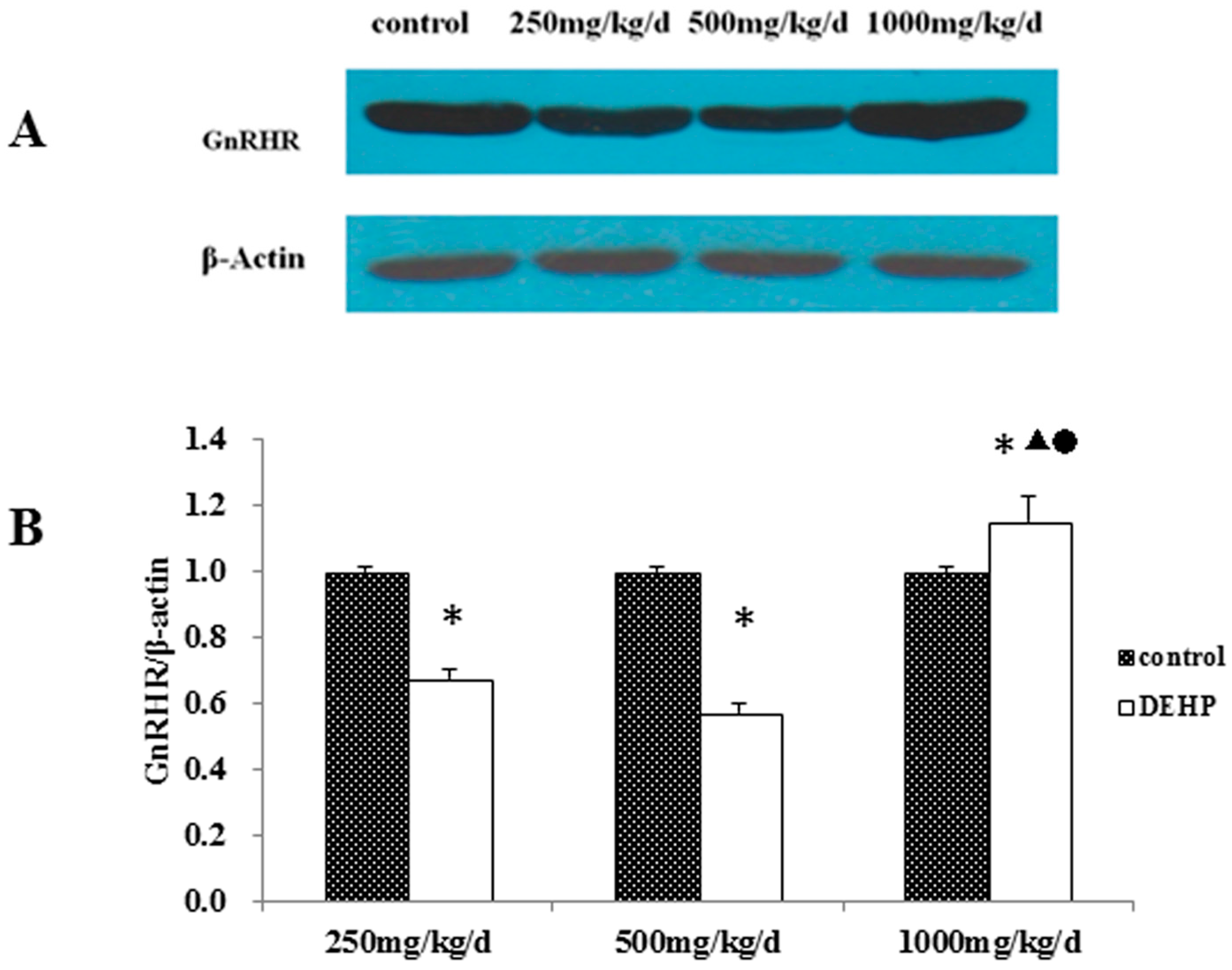
3.4. Gene and Protein Expression Levels of GnRHR in the Uterus
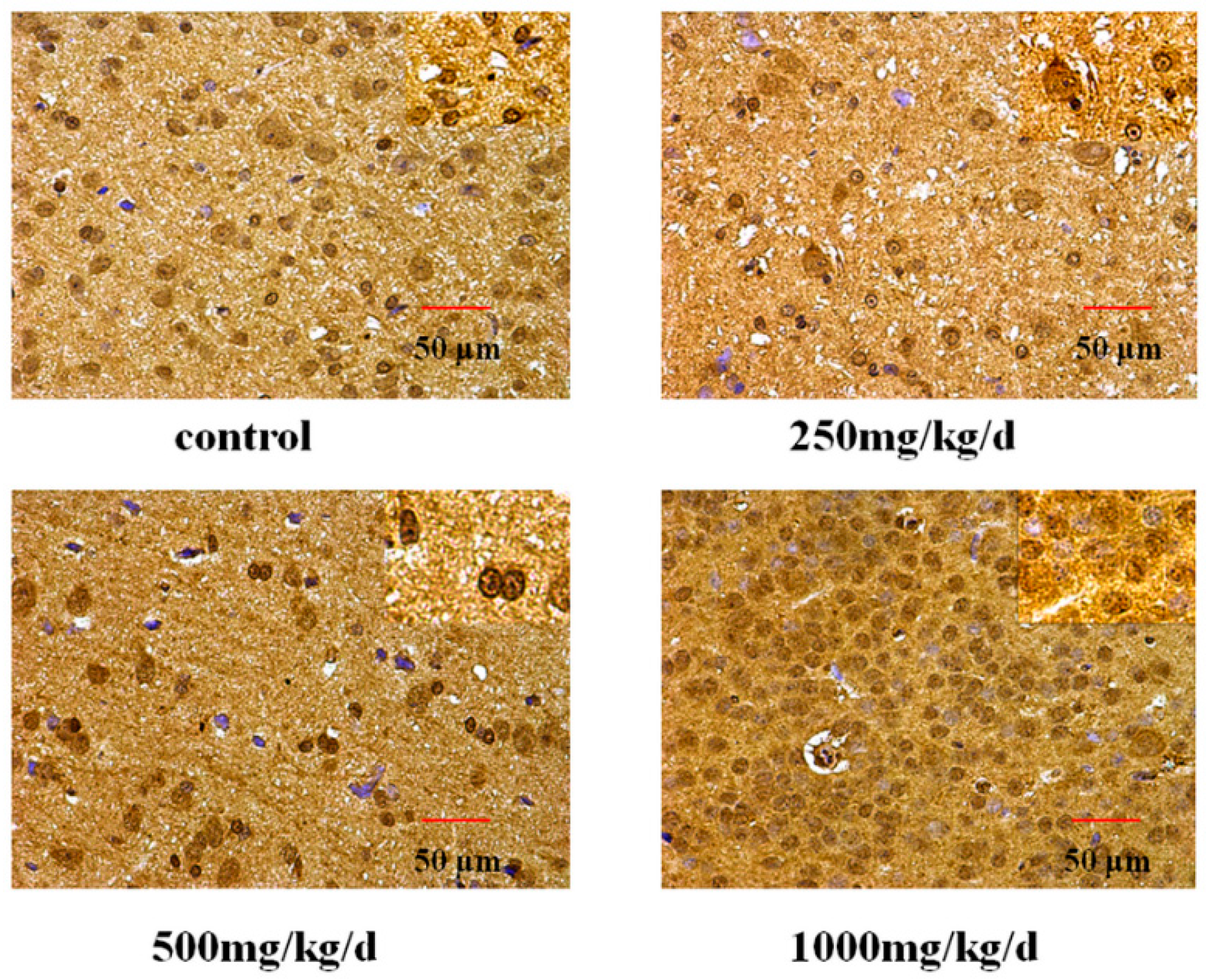
3.5. GnRH and GnRHR Immunohistochemistry
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al-Saleh, I.; Shinwari, N.; Alsabbaheen, A. Phthalates residues in plastic bottled waters. J. Toxicol. Sci. 2011, 36, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Bruning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Fasano, E.; Esposito, F.; Montuori, P.; Cocchieri, R.A. Di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DBP) exposure through diet in hospital patients. Food Chem. Toxicol. 2013, 51, 434–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastorakos, G.; Karoutsou, E.I.; Mizamtsidi, M.; Creatsas, G. The menace of endocrine disruptors on thyroid hormone physiology and their impact on intrauterine development. Endocrine 2007, 31, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, E. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J.P. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Earls, A.O.; Axford, I.P.; Braybrook, J.H. Gas chromatography-mass spectrometry determination of the migration of phthalate plasticisers from polyvinyl chloride toys and childcare articles. J. Chromatogr. A 2003, 983, 237–246. [Google Scholar] [CrossRef]
- Benson, R. Hazard to the developing male reproductive system from cumulative exposure to phthalate esters-dibutyl phthalate, diisobutyl phthalate, butylbenzyl phthalate, diethylhexyl phthalate, dipentyl phthalate, and diisononyl phthalate. Regul. Toxicol. Pharm. 2009, 53, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Latini, G. Potential hazards of exposure to di-(2-ethylhexyl)-phthalate in babies: A review. Biol. Neonate 2000, 78, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Adibi, J.J.; Whyatt, R.M.; Williams, P.L.; Calafat, A.M.; Camann, D.; Herrick, R.; Nelson, H.; Bhat, H.K.; Perera, F.A.; Silva, M.J.; et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008, 116, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hubal, E.A.C.; Little, J.C. Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: Sensitivity, uncertainty, and implications for biomonitoring. Environ. Health Perspect. 2010, 118, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, H.; Maddalena, R.L.; Singer, B.C.; Hodgson, A.T.; McKone, T.E. Indoor pollutants emitted by office equipment: A review of reported data and information needs. Atmos. Environ. 2008, 42, 1371–1388. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Lundgren, B.; Weschler, C.J.; Sigsgaard, T.; Hagerhed-Engman, L.; Sundell, J. Phthalates in indoor dust and their association with building characteristics. Environ. Health Perspect. 2005, 113, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Deutschle, T.; Reiter, R.; Butte, W.; Heinzow, B.; Keck, T.; Riechelmann, H. A controlled challenge study on di(2-ethylhexyl) phthalate (DEHP) in house dust and the immune response in human nasal mucosa of allergic subjects. Environ. Health Perspect. 2008, 116, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Kolarik, B.; Naydenov, K.; Larsson, M.; Bornehag, C.G.; Sundell, J. The association between phthalates in dust and allergic diseases among bulgarian children. Environ. Health Perspect. 2008, 116, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schripp, T.; Fauck, C.; Salthammer, T. Chamber studies on mass-transfer of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (dnbp) from emission sources into house dust. Atmos. Environ. 2010, 44, 2840–2845. [Google Scholar] [CrossRef]
- Geiss, O.; Tirendi, S.; Barrero-Moreno, J.; Kotzias, D. Investigation of volatile organic compounds and phthalates present in the cabin air of used private cars. Environ. Int. 2009, 35, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Angerer, J. Phthalates: Metabolism and exposure. Int. J. Androl. 2008, 31, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Calafat, A.M. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuhler, K. What are the sources of exposure to eight frequently used phthalic acid esters in europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, R.; Boekelheide, K.; Chapin, R.; Cunningham, M.; Faustman, E.; Foster, P.; Golub, M.; Henderson, R.; Hinberg, I.; Little, R.; et al. NTP center for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 2002, 16, 529–653. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Campioli, E.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. Fetal origin of endocrine dysfunction in the adult: The phthalate model. J. Steroid Biochem. 2013, 137, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Pocar, P.; Fiandanese, N.; Secchi, C.; Berrini, A.; Fischer, B.; Schmidt, J.S.; Schaedlich, K.; Borromeo, V. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 2012, 153, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.S.; Schaedlich, K.; Fiandanese, N.; Pocar, P.; Fischer, B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in c3h/n mice. Environ. Health Perspect. 2012, 120, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Singh, J.M.; Leslie, T.C.; Meachum, S.; Flaws, J.A.; Yao, H.H. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 2010, 242, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Agrawal, S.; Cook, T.J.; Knipp, G.T. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch. Toxicol. 2007, 81, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Knipp, G.T.; Cook, T.J. Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch. Toxicol. 2006, 80, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive summary to EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D. Exposure to environmental endocrine disruptors and child development. Arch. Pediatr. Adolesc. Med. 2012, 166, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Behie, A.M.; O’Donnell, M.H. Prenatal smoking and age at menarche: Influence of the prenatal environment on the timing of puberty. Hum. Reprod. 2015, 30, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, S.H.; Lee, H.W.; Chae, H.D.; Kim, C.H.; Kang, B.M. Increased viability of endometrial cells by in vitro treatment with di-(2-ethylhexyl) phthalate. Fertil. Steril. 2010, 94, 2413–2416. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chun, S.; Jang, J.Y.; Chae, H.D.; Kim, C.H.; Kang, B.M. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: A prospective case-control study. Fertil. Steril. 2011, 95, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Teitelbaum, S.L.; McGovern, K.; Windham, G.C.; Pinney, S.M.; Galvez, M.; Calafat, A.M.; Kushi, L.H.; Biro, F.M.; Breast, C.; et al. Phthalate exposure and pubertal development in a longitudinal study of U.S. girls. Hum. Reprod. 2014, 29, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, S.D.; Lei, X.; Ling, Y.S.; Luo, Y.; Liu, Q. Association of paes with precocious puberty in children: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2015, 12, 15254–15268. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.N.; Latronico, A.C.; Arnhold, I.J.; Mendonca, B.B. Update on the etiology, diagnosis and therapeutic management of sexual precocity. Arq. Bras. Endocrinol. Metabol. 2008, 52, 18–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larco, D.O.; Williams, M.; Schmidt, L.; Sabel, N.; Lange, J.; Woller, M.J.; Wu, T.J. Autoshortloop feedback regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by its metabolite, GnRH-(1-5). Endocrine 2015, 49, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.M.; Crowley, W.F., Jr. Gonadotropin-releasing hormone and its analogues. N. Engl. J. Med. 1991, 324, 93–103. [Google Scholar] [PubMed]
- Counis, R.; Laverriere, J.N.; Garrel, G.; Bleux, C.; Cohen-Tannoudji, J.; Lerrant, Y.; Kottler, M.L.; Magre, S. Gonadotropin-releasing hormone and the control of gonadotrope function. Reprod. Nutr. Dev. 2005, 45, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Hendy, G.N.; Percy, M.E.; Bichet, D.G.; Cole, D.E. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. 2014, 1175, 153–187. [Google Scholar] [PubMed]
- Roch, G.J.; Busby, E.R.; Sherwood, N.M. Gnrh receptors and peptides: Skating backward. Gen. Comp. Endocrinol. 2014, 209, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Basaran, Y.; Bolu, E.; Unal, H.U.; Sagkan, R.I.; Taslipinar, A.; Ozgurtas, T.; Musabak, U. Multiplex ligation dependent probe amplification analysis of KAL1, GnRH1, GnRHR, PROK2 and PROKR2 in male patients with idiopathic hypogonadotropic hypogonadism. Endokrynol. Pol. 2013, 64, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gianetti, E.; Hall, J.E.; Au, M.G.; Kaiser, U.B.; Quinton, R.; Stewart, J.A.; Metzger, D.L.; Pitteloud, N.; Mericq, V.; Merino, P.M.; et al. When genetic load does not correlate with phenotypic spectrum: Lessons from the GnRH receptor (GNRHR). J. Clin. Endocrinol. Metab. 2012, 97, E1798–E1807. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E. PCOS in adolescents. Best Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.C.; Liang, L.; Dong, G.P.; Zhao, Z.Y. Peripheral precocious puberty: A retrospective study for six years in Hangzhou, China. J. Paediatr. Child Health 2008, 44, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, D. Single and combined toxic effects of di-2-ethylhexyl phthalate and cypermethrin on fertility and development in the the prepubertal male rats. Wei Sheng Yan Jiu 2012, 41, 710–716. [Google Scholar] [PubMed]
- Kim, S.H.; Lee, H.W.; Kim, Y.H.; Koo, Y.H.; Chae, H.D.; Kim, C.H.; Lee, P.R.; Kang, B.M. Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum. Reprod. 2009, 24, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Schaedlich, K.; Schmidt, J.S.; Kwong, W.Y.; Sinclair, K.D.; Kurz, R.; Jahnke, H.G.; Fischer, B. Impact of di-ethylhexylphthalate exposure on metabolic programming in P19 ECC-derived cardiomyocytes. J. Appl. Toxicol. 2015, 35, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Sukhbaatar, U.; Mijiddorj, T.; Oride, A.; Kanasaki, H. Stimulation of delta subunit-containing GABAA receptor by DS1 increases GnRH receptor expression but reduces GnRH mRNA expression in GnRH-producing GT1–7 cells. Endocrine 2015, 49, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.S.; Chen, G.R.; Dong, Q.; Akingbemi, B.; Sottas, C.M.; Santos, M.; Sealfon, S.C.; Bernard, D.J.; Hardy, M.P. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J. Androl. 2007, 28, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Losa-Ward, S.M.; Todd, K.L.; McCaffrey, K.A.; Tsutsui, K.; Patisaul, H.B. Disrupted organization of RFamide pathways in the hypothalamus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol. Reprod. 2012, 87, 28. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Schricker, M.; Lakomek, M.; Strege, A.; Heiden, I.; Luft, H.; Munzel, U.; Wuttke, W.; Jarry, H. Autoregulation of the gonadotropin-releasing hormone (GnRH) system during puberty: Effects of antagonistic versus agonistic GnRH analogs in a female rat model. J. Endocrinol. 2001, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, N.; Zhu, J.; Yu, G.Y.; Guo, K.; Zhou, L.T.; Zheng, D.C.; Qu, X.F.; Huang, J.; Chen, X.; et al. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reprod. Toxicol. 2014, 46, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Samaniego, Y.A.; Cutrera, R.; Reynoso, R.; Cardoso, N.; Scacchi, P.; Moguilevsky, J.A.; Ponzo, O.J. Different effects by sex on hypothalamic-pituitary axis of prepubertal offspring rats produced by in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP). Neurotoxicology 2012, 33, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kaganovsky, E.; Rahimipour, S.; Ben-Aroya, N.; Okon, E.; Koch, Y. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: A putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res. 2002, 62, 1036–1044. [Google Scholar] [PubMed]
- Grundker, C.; Gunthert, A.R.; Millar, R.P.; Emons, G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J. Clin. Endocr. Metab. 2002, 87, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Chatzaki, E.; Bax, C.M.; Eidne, K.A.; Anderson, L.; Grudzinskas, J.G.; Gallagher, C.J. The expression of gonadotropin-releasing hormone and its receptor in endometrial cancer, and its relevance as an autocrine growth factor. Cancer Res. 1996, 56, 2059–2065. [Google Scholar] [PubMed]
- Zhang, X.; Healy, C.; Nothnick, W.B. Estrogen suppresses expression of the matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with kazal motifs (RECK) within the mouse uterus. Endocrine 2012, 42, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, S.; Ihm, H.J.; Oh, Y.S.; Heo, S.H.; Chun, S.; Im, H.; Chae, H.D.; Kim, C.H.; Kang, B.M. Possible role of phthalate in the pathogenesis of endometriosis: In vitro, animal, and human data. J. Clin. Endocrinol. Metab. 2015, 100, E1502–E1511. [Google Scholar] [CrossRef] [PubMed]
- Fiorio, P.; Rosaia De Santis, L.; Cuoco, C.; Gimelli, G.; Gastaldi, R.; Bonatti, F.; Ravazzolo, R.; Bocciardi, R. Hypogonadotropic hypogonadism in a trisomy X carrier: Phenotype description and genotype correlation. Gynecol. Endocrinol. 2015, 32, 14–17. [Google Scholar] [CrossRef] [PubMed]


| Gene | Direction | Sequence |
|---|---|---|
| GNRH | Forward | TCCAGCCAGCACTGGTCCTA |
| Reverse | GGGTTCTGCCATTTGATCCTC | |
| GnRHR | Forward | TCACCTTCAGCTGCCTGTTCA |
| Reverse | CTCAGCCGTGCTCTTGGGATA | |
| β-actin | Forward | CCCATTGAACACGGCATTG |
| Reverse | GGTACGACCAGAGGCATACA |
| Groups | n | Time (Weeks) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Control | 12 | 13.33 ± 3.01 | 13.66 ± 4.20 | 14.60 ± 2.86 | 14.53 ± 3.69 |
| 250 mg/kg/d | 12 | 17.00 ± 2.44 * | 18.82 ± 3.10 * | 17.77 ± 3.34 | 16.20 ± 3.95 |
| 500 mg/kg/d | 12 | 14.25 ± 1.23 ▲ | 15.48 ± 3.38 | 18.39 ± 2.97 | 17.15 ± 2.93 |
| 1000 mg/kg/d | 12 | 16.55 ± 2.22 * | 17.82 ± 4.32 | 22.28 ± 2.37 * | 18.53 ± 1.74 * |
| Groups | n | Time (Weeks) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Control | 12 | 76.87 ± 8.43 | 106.73 ± 9.25 | 134.00 ± 6.85 | 152.12 ± 3.89 |
| 250 mg/kg/d | 12 | 8 85.12 ± 9.31 | 116.58 ± 8.70 * | 144.27 ± 7.40 * | 164.69 ± 8.05 * |
| 500 mg/kg/d | 12 | 87.17 ± 9.75 | 117.50 ± 7.46 * | 143.74 ± 8.89 * | 168.52 ± 7.59 * |
| 1000 mg/kg/d | 12 | 90.43 ± 10.91 * | 122.05 ± 7.88 * | 149.02 ± 7.35 * | 170.16 ± 6.46 * |
| Groups | n | Time (Weeks) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Control | 12 | 17.12 ± 3.08 | 25.62 ± 7.22 | 29.21 ± 7.82 | 25.10 ± 5.87 |
| 250 mg/kg/d | 12 | 18.92 ± 4.21 | 26.04 ± 5.20 | 26.83 ± 8.20 | 32.85 ± 1.79 * |
| 500 mg/kg/d | 12 | 22.38 ± 4.25 * | 27.30 ± 3.63 | 29.26 ± 6.67 | 35.48 ± 5.80 * |
| 1000 mg/kg/d | 12 | 17.98 ± 1.32 ● | 17.46 ± 3.34 *,▲,● | 22.80 ± 8.94 | 23.76 ± 3.35 ▲,● |
| Groups | Dosage, mg/kg/d | MOD (10−2) | IOD (×104) |
|---|---|---|---|
| Control | - | 15.322 ± 0.853 | 66.195 ± 6.038 |
| DEHP | 250 | 17.081 ± 0.728 b | 78.000 ± 4.794 b |
| 500 | 16.242 ± 0.537 b | 72.200 ± 6.123 b | |
| 1000 | 16.492 ± 0.521 b | 74.340 ± 7.962 b |
| Groups | Dosage, mg/kg/d | MOD (10−2) | IOD (×104) |
|---|---|---|---|
| Control | - | 12.941 ± 1.024 | 44.028 ± 0.868 |
| DEHP | 250 | 16.096 ± 0.711 b | 50.458 ± 4.719 b |
| 500 | 14.893 ± 0.563 b,c | 48.971 ± 1.825 b,c | |
| 1000 | 15.748 ± 0.160 b | 52.083 ± 7.565 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Jia, Y.; Zhou, L.; Wang, Q.; Sun, D.; Xu, J.; Wu, J.; Chen, H.; Xu, F.; Ye, L. Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus–Uterus in Pubertal Female Rats. Int. J. Environ. Res. Public Health 2016, 13, 1130. https://doi.org/10.3390/ijerph13111130
Liu T, Jia Y, Zhou L, Wang Q, Sun D, Xu J, Wu J, Chen H, Xu F, Ye L. Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus–Uterus in Pubertal Female Rats. International Journal of Environmental Research and Public Health. 2016; 13(11):1130. https://doi.org/10.3390/ijerph13111130
Chicago/Turabian StyleLiu, Te, Yiyang Jia, Liting Zhou, Qi Wang, Di Sun, Jin Xu, Juan Wu, Huaiji Chen, Feng Xu, and Lin Ye. 2016. "Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus–Uterus in Pubertal Female Rats" International Journal of Environmental Research and Public Health 13, no. 11: 1130. https://doi.org/10.3390/ijerph13111130
APA StyleLiu, T., Jia, Y., Zhou, L., Wang, Q., Sun, D., Xu, J., Wu, J., Chen, H., Xu, F., & Ye, L. (2016). Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus–Uterus in Pubertal Female Rats. International Journal of Environmental Research and Public Health, 13(11), 1130. https://doi.org/10.3390/ijerph13111130





