Association of Urogenital Symptoms with History of Water Contact in Young Women in Areas Endemic for S. haematobium. A Cross-Sectional Study in Rural South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
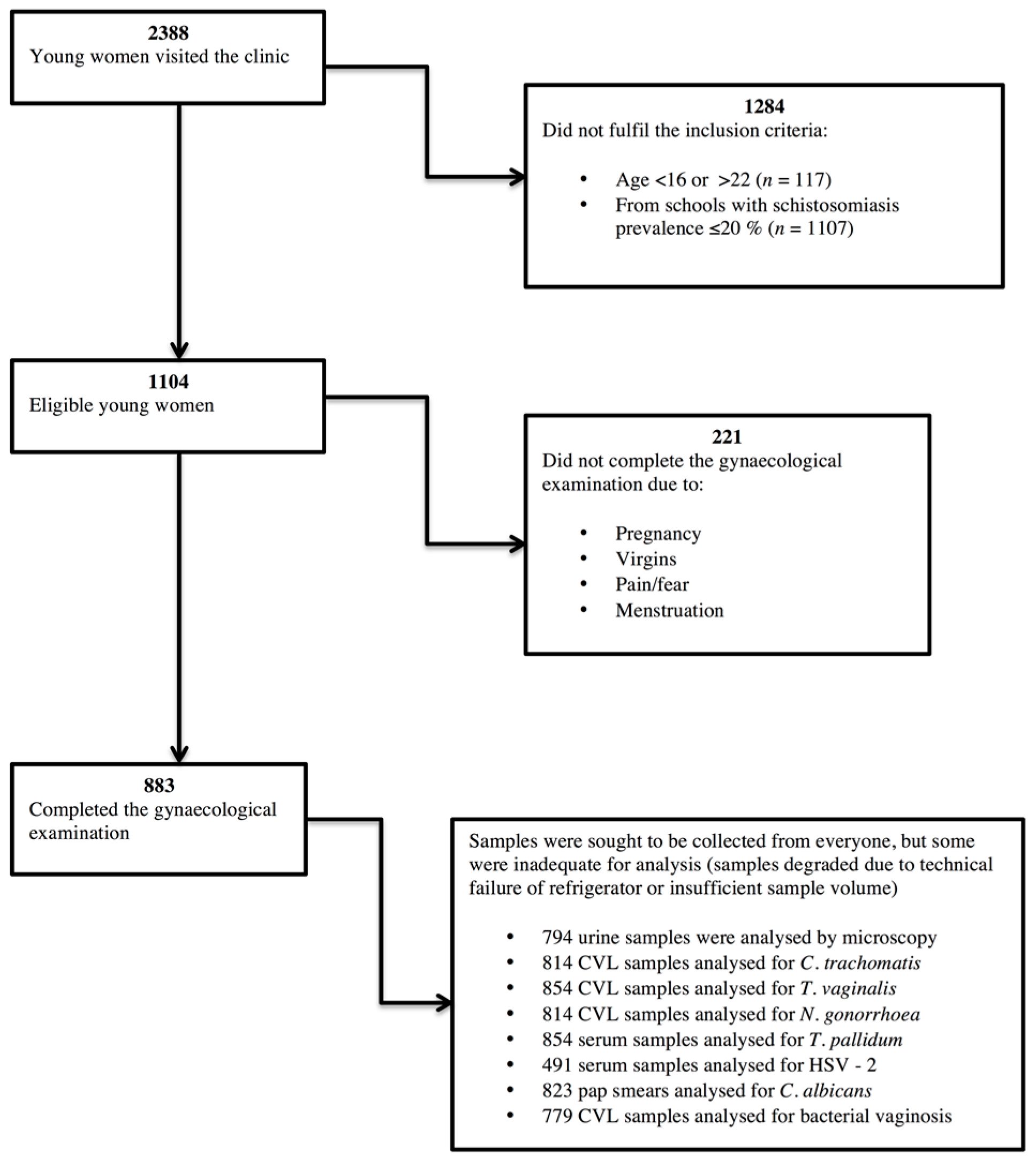
2.2. Recruiting Participants
2.3. Questionnaire
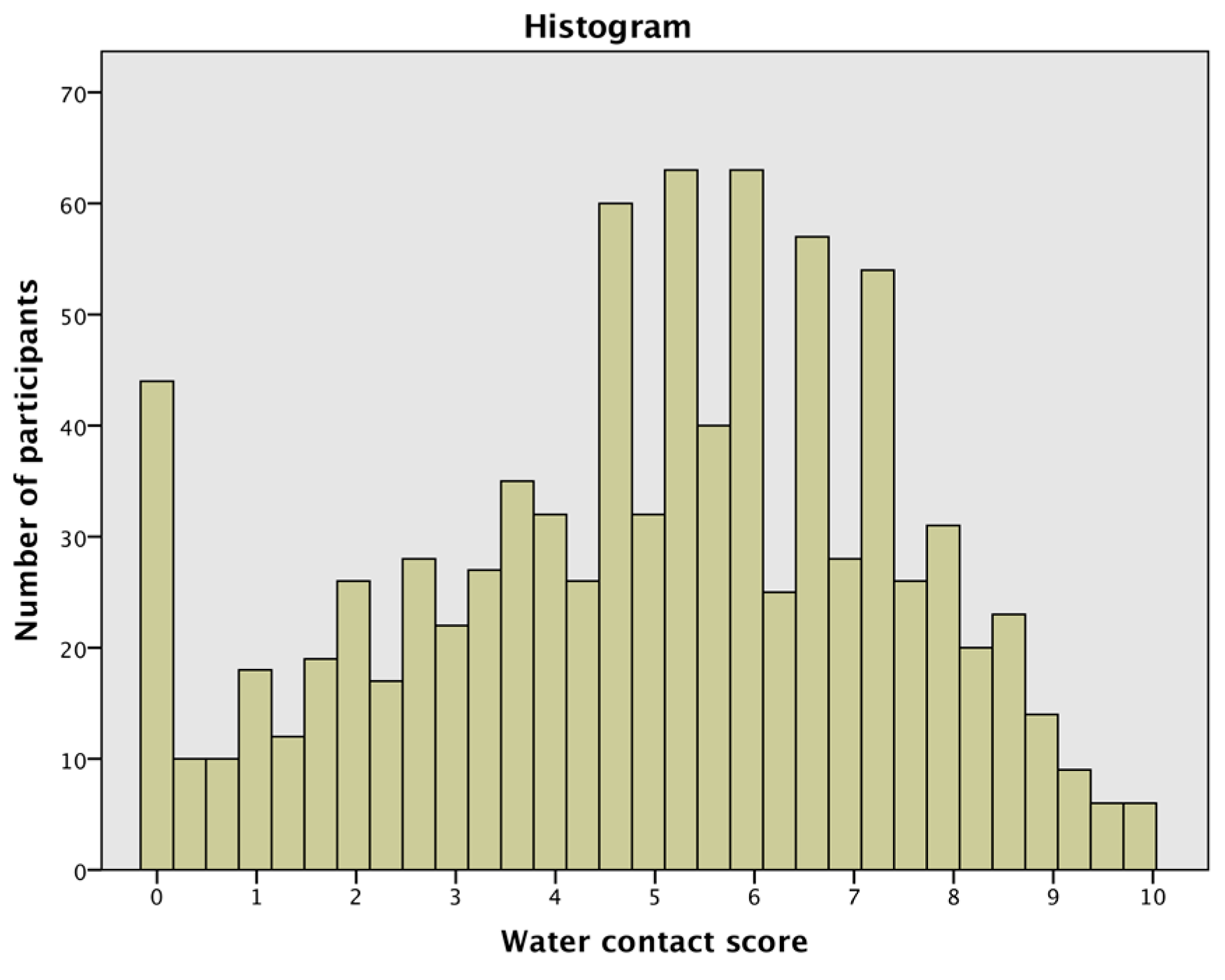
2.4. Water Contact Score
2.5. Sample Collection and Laboratory Analyses
2.6. Ethical Considerations
2.7. Statistical Analyses
3. Results
3.1. General Characteristics of the Study Population
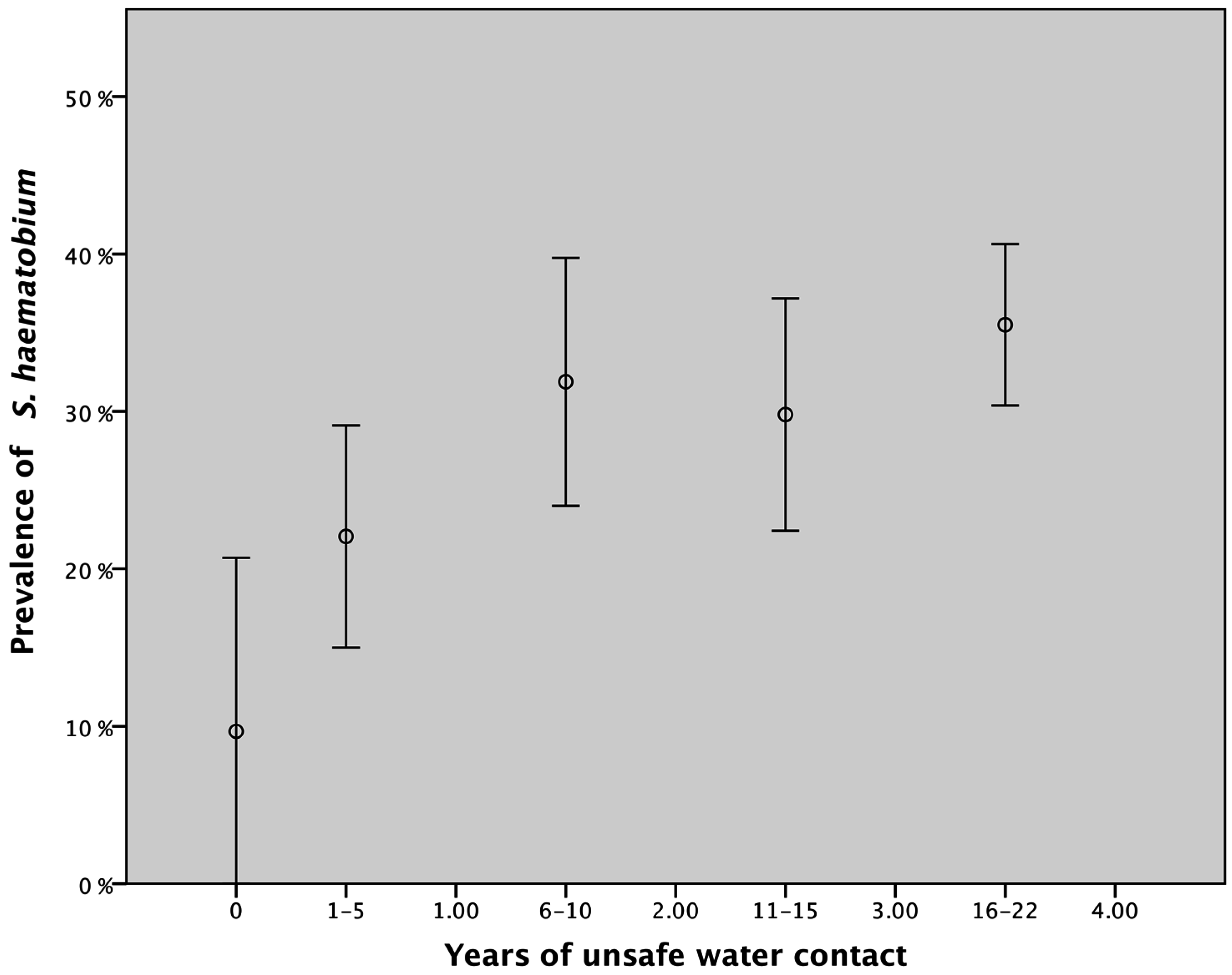
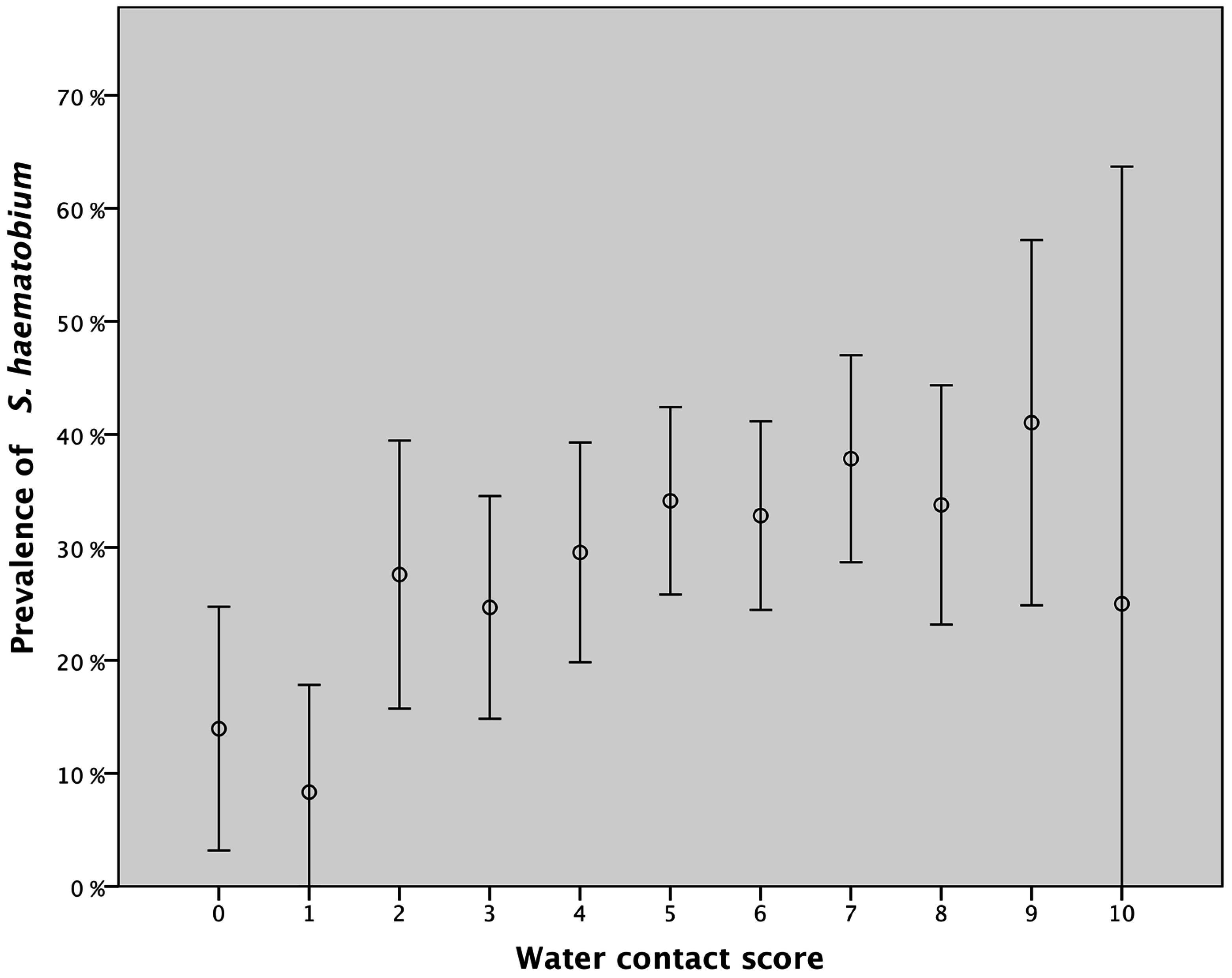
3.2. Water Contact and Urinary Schistosomiasis
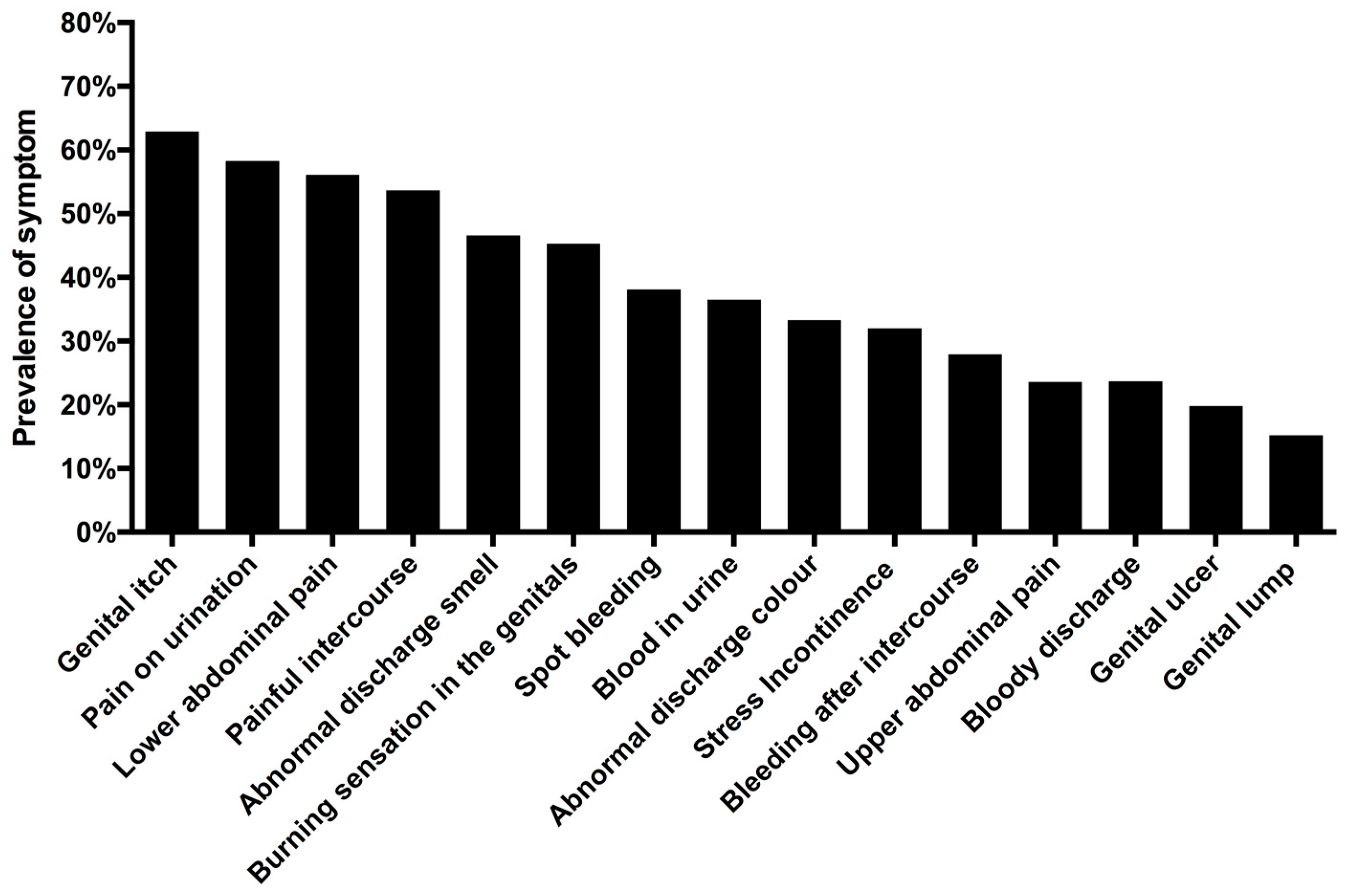
3.3. Unsafe Water Contact and Self-Reported Urogenital Symptoms
3.4. Current Unsafe Water Contact and Urogenital Symptoms
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hotez, P.J.; Fenwick, A.; Kjetland, E.F. Africa’s 32 cents solution for HIV/AIDS. PLoS Negl. Trop. Dis. 2009, 3, e430. [Google Scholar] [CrossRef] [PubMed]
- Kjetland, E.F.; Leutscher, P.D.; Ndhlovu, P.D. A review of female genital schistosomiasis. Trends Parasitol. 2012, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Boissier, J.; Grech-Angelini, S.; Webster, B.L.; Allienne, J.F.; Huyse, T.; Mas-Coma, S.; Toulza, E.; Barre-Cardi, H.; Rollinson, D.; Kincaid-Smith, J.; et al. Outbreak of urogenital schistosomiasis in Corsica (France): An epidemiological case study. Lancet Infect. Dis. 2016, 16, 971–979. [Google Scholar] [CrossRef]
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The global status of schistosomiasis and its control. Acta Trop. 2000, 77, 41–51. [Google Scholar] [CrossRef]
- Nour, N.M. Schistosomiasis: Health effects on women. Rev. Obstet. Gynecol. 2010, 3, 28–32. [Google Scholar] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Kjetland, E.F.; Ndhlovu, P.D.; Mduluza, T.; Gomo, E.; Gwanzura, L.; Mason, P.R.; Kurewa, E.N.; Midzi, N.; Friis, H.; Gundersen, S.G. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am. J. Trop. Med. Hyg. 2005, 72, 311–319. [Google Scholar] [PubMed]
- Downs, J.A.; van Dam, G.J.; Changalucha, J.M.; Corstjens, P.L.; Peck, R.N.; de Dood, C.J.; Bang, H.; Andreasen, A.; Kalluvya, S.E.; van Lieshout, L.; et al. Association of schistosomiasis and HIV infection in Tanzania. Am. J. Trop. Med. Hyg. 2012, 87, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Kjetland, E.F.; Ndhlovu, P.D.; Gomo, E.; Mduluza, T.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Sandvik, L.; Friis, H.; Gundersen, S.G. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 2006, 20, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, H.; Brooker, S.; Lwambo, N.J.; Siza, J.E.; Bundy, D.A. The performance of school-based questionnaires of reported blood in urine in diagnosing Schistosoma haematobium infection: Patterns by age and sex. Trop. Med. Int. Health 1999, 4, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Lengeler, C.; de Savigny, D.; Mshinda, H.; Mayombana, C.; Tayari, S.; Hatz, C.; Degremont, A.; Tanner, M. Community-based questionnaires and health statistics as tools for the cost-efficient identification of communities at risk of urinary schistosomiasis. Int. J. Epidemiol. 1991, 20, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Lengeler, C.; Utzinger, J.; Tanner, M. Questionnaires for rapid screening of schistosomiasis in Sub-Saharan Africa. Bull. World Health Organ. 2002, 80, 235–242. [Google Scholar] [PubMed]
- Hegertun, I.E.; Sulheim Gundersen, K.M.; Kleppa, E.; Zulu, S.G.; Gundersen, S.G.; Taylor, M.; Kvalsvig, J.D.; Kjetland, E.F. S. Haematobium as a common cause of genital morbidity in girls: A cross-sectional study of children in South Africa. PLoS Negl. Trop. Dis. 2013, 7, e2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, P.; Taylor, M.; Zulu, S.G.; Gundersen, S.G.; Verweij, J.J.; Hoekstra, P.; Brienen, E.A.; Kleppa, E.; Kjetland, E.F.; van Lieshout, L. Real-time polymerase chain reaction for detection of schistosoma DNA in small-volume urine samples reflects focal distribution of urogenital schistosomiasis in primary school girls in Kwazulu Natal, South Africa. Am. J. Trop. Med. Hyg. 2014, 90, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Sama, M.T.; Oyono, E.; Ratard, R.C. High risk behaviours and schistosomiasis infection in Kumba, South-West Province, Cameroon. Int. J. Environ. Res. Public Health 2007, 4, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, A.; Bethony, J.; Fraga, L.A.; LoVerde, P.T.; Correa-Oliveira, R.; Kloos, H. Exposure to Schistosoma mansoni infection in a rural area of Brazil. I: Water contact. Trop. Med. Int. Health 2001, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Holmen, S.D.; Kleppa, E.; Lillebo, K.; Pillay, P.; van Lieshout, L.; Taylor, M.; Albregtsen, F.; Vennervald, B.J.; Onsrud, M.; Kjetland, E.F. The first step toward diagnosing female genital schistosomiasis by computer image analysis. Am. J. Trop. Med. Hyg. 2015, 93, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Becker, S.L.; van Lieshout, L.; van Dam, G.J.; Knopp, S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015, 21, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Kleppa, E.; Ramsuran, V.; Zulu, S.; Karlsen, G.H.; Bere, A.; Passmore, J.A.; Ndhlovu, P.; Lillebo, K.; Holmen, S.D.; Onsrud, M.; et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS ONE 2014, 9, e98593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodley, D.; Moodley, P.; Sebitloane, M.; Soowamber, D.; McNaughton-Reyes, H.L.; Groves, A.K.; Maman, S. High prevalence and incidence of asymptomatic sexually transmitted infections during pregnancy and postdelivery in Kwazulu Natal, south Africa. Sex. Transm. Dis. 2015, 42, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kleppa, E.; Holmen, S.D.; Lillebo, K.; Kjetland, E.F.; Gundersen, S.G.; Taylor, M.; Moodley, P.; Onsrud, M. Cervical ectopy: Associations with sexually transmitted infections and HIV. A cross-sectional study of high school students in rural South Africa. Sex. Transm. Infect. 2015, 91, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Joesoef, M.R.; Hillier, S.L.; Josodiwondo, S.; Linnan, M. Reproducibility of a scoring system for gram stain diagnosis of bacterial vaginosis. J. Clin. Microbiol. 1991, 29, 1730–1731. [Google Scholar] [PubMed]
- Mlisana, K.; Naicker, N.; Werner, L.; Roberts, L.; van Loggerenberg, F.; Baxter, C.; Passmore, J.A.; Grobler, A.C.; Sturm, A.W.; Williamson, C.; et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J. Infect. Dis. 2012, 206, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Holmen, S.; Galappaththi-Arachchige, H.N.; Kleppa, E.; Pillay, P.; Naicker, T.; Taylor, M.; Onsrud, M.; Kjetland, E.F.; Albregtsen, F. Characteristics of blood vessels in female genital schistosomiasis: Paving the way for objective diagnostics at the point of care. PLoS Negl. Trop. Dis. 2016, 10, e0004628. [Google Scholar] [CrossRef] [PubMed]
- Audisio, T.; Pigini, T.; de Riutort, S.V.; Schindler, L.; Ozan, M.; Tocalli, C.; Bertolotto, P. Validity of the Papanicolaou smear in the diagnosis of Candida spp., Trichomonas vaginalis, and bacterial vaginosis. J. Low. Genit. Tract Dis. 2001, 5, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; van Lieshout, L.; Taylor, M.; Sebitloane, M.; Zulu, S.G.; Kleppa, E.; Roald, B.; Kjetland, E.F. Cervical cytology as a diagnostic tool for female genital schistosomiasis: Correlation to cervical atypia and schistosoma polymerase chain reaction. Cytojournal 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, P.M.; Holmen, S.D.; Gundersen, S.G.; Roald, B.; Kjetland, E.F. HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am. J. Trop. Med. Hyg. 2011, 85, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Kjetland, E.F.; Kurewa, E.N.; Ndhlovu, P.D.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Gomo, E.; Sandvik, L.; Mduluza, T.; Friis, H.; et al. Female genital schistosomiasis—A differential diagnosis to sexually transmitted disease: Genital itch and vaginal discharge as indicators of genital Schistosoma haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Trop. Med. Int. Health 2008, 13, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Leutscher, P.D.; Ramarokoto, C.E.; Hoffmann, S.; Jensen, J.S.; Ramaniraka, V.; Randrianasolo, B.; Raharisolo, C.; Migliani, R.; Christensen, N. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin. Infect. Dis. 2008, 47, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Poggensee, G.; Kiwelu, I.; Weger, V.; Goppner, D.; Diedrich, T.; Krantz, I.; Feldmeier, H. Female genital schistosomiasis of the lower genital tract: Prevalence and disease-associated morbidity in Northern Tanzania. J. Infect. Dis. 2000, 181, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Leutscher, P.D.; Ravaoalimalala, V.E.; Raharisolo, C.; Ramarokoto, C.E.; Rasendramino, M.; Raobelison, A.; Vennervald, B.; Esterre, P.; Feldmeier, H. Clinical findings in female genital schistosomiasis in Madagascar. Trop. Med. Int. Health 1998, 3, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kjetland, E.F.; Poggensee, G.; Helling-Giese, G.; Richter, J.; Sjaastad, A.; Chitsulo, L.; Kumwenda, N.; Gundersen, S.G.; Krantz, I.; Feldmeier, H. Female genital schistosomiasis due to Schistosoma haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Trop. 1996, 62, 239–255. [Google Scholar] [CrossRef]
- Schur, N.; Vounatsou, P.; Utzinger, J. Determining treatment needs at different spatial scales using geostatistical model-based risk estimates of schistosomiasis. PLoS Negl. Trop. Dis. 2012, 6, e1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloos, H.; Rodrigues, J.C.; Pereira, W.R.; Velasquez-Melendez, G.; Loverde, P.; Oliveira, R.C.; Gazzinelli, A. Combined methods for the study of water contact behavior in a rural schistosomiasis-endemic area in Brazil. Acta Trop. 2006, 97, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.J.; Chavez, S.; Feringa, B.; Chiappe, M.; Li, W.; Jansen, K.U.; Carcamo, C.; Holmes, K.K. Reproductive tract infections in rural women from the highlands, jungle, and coastal regions of Peru. Bull. World Health Organ. 2004, 82, 483–492. [Google Scholar] [PubMed]

| Activity | Time Range (h) | Median Body Surface (%) | Activity Risk Coefficient |
|---|---|---|---|
| Playing | 1–3 | 100 | 5 |
| Bathing | 1–3 | 100 | 5 |
| Washing blankets | 3–5 | 35 | 4 |
| Laundry | 1–3 | 35 | 3 |
| Fishing | 1–3 | 35 | 3 |
| Collecting water | ≤1 | 20 | 1 |
| Crossing | ≤1 | 25 | 1 |
| Age | Number of Pupils (%) |
|---|---|
| 16–17 | 214 (24.2) |
| 18–19 | 397 (44.9) |
| 20–22 | 272 (30.8) |
| Total | 883 |
| Symptom | Adjusted OR | 95% CI | p Value a |
|---|---|---|---|
| Red urine | |||
| Water contact score b | 1.16 | 1.09–1.24 | <0.001 |
| Neisseria gonorrhea c | 1.65 | 1.07–2.55 | 0.023 |
| Stress incontinence | |||
| Water contact score b | 1.11 | 1.04–1.18 | 0.001 |
| Neisseria gonorrhea c | 1.70 | 1.10–2.63 | 0.016 |
| Burning sensation in the genitals | |||
| Water contact score b | 1.09 | 1.03–1.15 | 0.005 |
| Neisseria gonorrhea c | 1.83 | 1.19–2.81 | 0.006 |
| Spot bleeding d | |||
| Water contact score b | 1.10 | 1.02–1.18 | 0.012 |
| Abnormal discharge smell | |||
| Water contact score b | 1.07 | 1.01–1.14 | 0.018 |
| Neisseria gonorrhea c | 1.49 | 0.97–2.29 | 0.070 |
| Bloody discharge d | |||
| Water contact score b | 1.11 | 1.02–1.21 | 0.020 |
| Lower abdominal pain e | |||
| Water contact score b | 1.07 | 1.00–1.14 | 0.028 |
| Neisseria gonorrhea c | 1.46 | 0.91–2.35 | 0.119 |
| Genital ulcer | |||
| Water contact score b | 1.08 | 1.00–1.16 | 0.038 |
| Neisseria gonorrhea c | 1.63 | 1.01–2.64 | 0.045 |
| Symptom | Adjusted OR | 95% CI | p Value a |
|---|---|---|---|
| Red urine | |||
| Water contact score b | 1.19 | 1.07–1.32 | 0.001 |
| Neisseria gonorrhoea c | 1.46 | 0.75–2.83 | 0.266 |
| Pain on urination | |||
| Water contact score b | 1.15 | 1.04–1.27 | 0.006 |
| Neisseria gonorrhoea c | 1.93 | 0.93–4.01 | 0.078 |
| Burning sensation in the genitals | |||
| Water contact score b | 1.17 | 1.06–1.30 | 0.002 |
| Neisseria gonorrhoea c | 1.99 | 1.02–3.88 | 0.043 |
| Stress incontinence | |||
| Water contact score b | 1.17 | 1.05–1.30 | 0.004 |
| Neisseria gonorrhoea c | 1.58 | 0.82–3.07 | 0.175 |
| Spot bleeding d | |||
| Water contact score b | 1.18 | 1.05–1.33 | 0.005 |
| Post coital bleeding d | |||
| Water contact score b | 1.18 | 1.04–1.34 | 0.012 |
| Abnormal discharge smell | |||
| Water contact score b | 1.11 | 1.01–1.22 | 0.027 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galappaththi-Arachchige, H.N.; Amlie Hegertun, I.E.; Holmen, S.; Qvigstad, E.; Kleppa, E.; Sebitloane, M.; Ndhlovu, P.D.; Vennervald, B.J.; Gundersen, S.G.; Taylor, M.; et al. Association of Urogenital Symptoms with History of Water Contact in Young Women in Areas Endemic for S. haematobium. A Cross-Sectional Study in Rural South Africa. Int. J. Environ. Res. Public Health 2016, 13, 1135. https://doi.org/10.3390/ijerph13111135
Galappaththi-Arachchige HN, Amlie Hegertun IE, Holmen S, Qvigstad E, Kleppa E, Sebitloane M, Ndhlovu PD, Vennervald BJ, Gundersen SG, Taylor M, et al. Association of Urogenital Symptoms with History of Water Contact in Young Women in Areas Endemic for S. haematobium. A Cross-Sectional Study in Rural South Africa. International Journal of Environmental Research and Public Health. 2016; 13(11):1135. https://doi.org/10.3390/ijerph13111135
Chicago/Turabian StyleGalappaththi-Arachchige, Hashini Nilushika, Ingrid Elise Amlie Hegertun, Sigve Holmen, Erik Qvigstad, Elisabeth Kleppa, Motshedisi Sebitloane, Patricia Doris Ndhlovu, Birgitte Jyding Vennervald, Svein Gunnar Gundersen, Myra Taylor, and et al. 2016. "Association of Urogenital Symptoms with History of Water Contact in Young Women in Areas Endemic for S. haematobium. A Cross-Sectional Study in Rural South Africa" International Journal of Environmental Research and Public Health 13, no. 11: 1135. https://doi.org/10.3390/ijerph13111135






