Overt Primary Hypothyroidism in an Industrial Area in São Paulo, Brazil: The Impact of Public Disclosure
Abstract
:1. Introduction
2. Methods
2.1. Ethical Statement
2.2. Subjects
2.3. Sample Collection
2.4. Thyroid Hormones Measurement
2.5. Sonographic Scan
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gist, G.L.; Burg, J.R. Trichloroethylene: A review of the literature from a health effects perspective. Toxicol. Ind. Health 1995, 11, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Tremont, G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007, 32, 49–65. [Google Scholar] [PubMed]
- Brent, G.A. The molecular basis of thyroid hormone action. N. Engl. J. Med. 1994, 331, 847–853. [Google Scholar] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Wolber, K. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012, 22, 1200–1235. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.H. Thyroid hormones and cognitive function. Expert Rev. Endocrinol. Metab. 2012, 7, 365–367. [Google Scholar] [CrossRef]
- Weber, G.; Vigone, M.C.; Stroppa, L.; Chiumello, G. Thyroid function and puberty. J. Pediatr. Endocrinol. Metab. 2003, 16, 253–257. [Google Scholar] [PubMed]
- Vanderpump, M.P.J.; Tunbridge, W.M.G.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Grimley, E.J.; Hasan, D.M.; Rodgers, H.; Tunbridge, F.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef]
- Wang, C.; Crapo, L.M. The epidemiology of thyroid disease and implications for screening. Endocrinol. Metab. Clin. N. Am. 1997, 26, 189–218. [Google Scholar] [CrossRef]
- Tunbridge, W.M.G.; Vanderpump, M.P.J. Population screening for autoimmune thyroid disease. Endocrinol. Metab. Clin. N. Am. 2000, 29, 239–253. [Google Scholar] [CrossRef]
- Dayan, C.M.; Daniels, G.H. Chronic autoimmune thyroiditis. N. Engl. J. Med. 1996, 335, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Trimarchi, F. Changed presentation of Hashimoto’s Thyroiditis in north-eastern Sicily and Calabria (southern Italy) based on a 31-year experience. Thyroid 2008, 18, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Rossi, R.T.; Bonaffini, O.; Scisca, C.; Altavilla, G.; Calbo, L.; Rosano, A.; Sindoni, A.; Trimarchi, F.; Benvenga, S. Increased annual frequency of Hashimoto’s Thyroiditis between years 1988 and 2007 at a cytological unit of Sicily. Ann. Endocrinol. 2010, 71, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.M.; Griffin, J.M.; Pumford, N.R. Trichloroethylene activates CD4+ T cells: Potential role in an autoimmune response. Drug Metab. Rev. 1999, 31, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Selgrade, M.K. Immunotoxicity: The risk is real. Toxicol. Sci. 2007, 100, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Bahn, A.K.; Mills, J.L.; Synder, P.J.; Gann, P.H.; Houten, L.; Bialik, O.; Hollmann, L.; Utiger, R.D. Hypothyroidism in workers exposed to polybrominated biphenyls. N. Engl. J. Med. 1980, 302, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Environmental exposures and autoimmune thyroid disease. Thyroid 2010, 20, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Burek, C.L.; Talor, M.V. Environmental triggers of autoimmune thyroiditis. J. Autoimmun. 2009, 33, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chiu, H.F.; Tsai, S.S.; Chang, C.C.; Chuang, H.Y. Increased risk of preterm delivery in areas with cancer mortality problems from petrochemical complex. Environ. Res. 2002, 69, 195–200. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Ward-Smith, P.; Morantz, R.A.; Tian, C.; Schmelzie, K.H.; Mayo, M.S.; Chin, T.D. Brain cancer in a residential area bordering on an oil refinery. Neuroepidemiology 2003, 22, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zaccarelli-Marino, M.A. Chronic autoimmune thyroiditis in industrial areas in Brazil: A 15 year survey. J. Clin. Immun. 2012, 32, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.U.; Campos, R.A.G.; Silva, M.A.F.R.; Panachão, M.R.I.; Moraes, J.C.; Waissmann, W.; Chacra, A.R.; Maeda, M.Y.S.; Rodrigues, R.S.M.; Belchor, L.G.; et al. Can living in the surroundings of a petrochemical complex be a risk factor for autoimmune thyroid disease? Environ. Res. 2010, 110, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Esteves, R.Z.; Kasamatsu, T.S.; Kunii, I.S.; Furuzawa, G.K.; Vieira, J.G.H.; Maciel, R.M.B. Development of a semi-automated method of measuring urinary iodine and its application in epidemiological studies in Brazilian schoolchildren. Braz. Arch. Endocrinol. Metab. 2007, 51, 1477–1484. [Google Scholar] [CrossRef]
- Zaccarelli-Marino, M.A.; Martins, L.C.; Esteves, R.Z.; Kasamatsu, T.S.; Maciel, R.M.B. Urinary iodine in patients with auto-immune thyroid disorders in Santo André, SP, is comparable to normal controls and has been steady for the last 10 years. Braz. Arch. Endocrinol. Metab. 2009, 53, 55–63. [Google Scholar]
- Agresti, A. Categorical Data Analysis, 3rd ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 339–376. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statiscal Computing: Vienna, Austria, 2016. [Google Scholar]
- Landrigan, P.G. Children as a vulnerable population. Int. J. Occup. Med. Environ. Health 2004, 17, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Radetti, G.; Gottardi, E.; Bona, G.; Corrias, A.; Salardi, S.; Loche, S. Study group for thyroid diseases of the Italian society for pediatric endocrinology and diabetes (SIEDP/ISPED). The natural history of euthyroid Hashimoto’s Thyroiditis in children. J. Pediatr. 2006, 149, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.J.; Tunbridge, W.M.G. The epidemiology of thyroid diseases. In Werner and Ingbar’s the Thyroid: A Fundamental and Clinical Text; Braverman, L.E., Utiger, R.D., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2000; pp. 467–473. [Google Scholar]
- Tunbridge, W.M.G.; Evered, D.C.; Hall, R.; Appleton, D.; Brewis, M.; Clark, F.; Grimley, E.J.; Young, E.; Bird, T.; Smith, A. The spectrum of thyroid disease in a community: The Whickham Survey. Clin. Endocrinol. 1997, 7, 481–493. [Google Scholar] [CrossRef]
- Canaris, G.J.; Manowitz, N.R.; Mayor, G.; Ridgway, E.C. The Colorado thyroid disease prevalence study. Arch. Int. Med. 2000, 160, 526–534. [Google Scholar] [CrossRef]
- Aoki, Y.; Belin, R.M.; Clickner, R.; Jeffries, R.; Phillips, L.; Mahaffey, K.R. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007, 17, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G.; Staehling, N.W.; Flanders, D.F.W.; Hannon, H.W.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E.; Serum, T.S.H. T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Antonelli, A.; Vita, R. Thyroid nodules and thyroid autoimmunity in the context of environmental pollution. Rev. Endocrinol. Metab. Disord. 2015, 16, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Latina, A.; Baratta, R.; Burgio, G.; Gullo, D.; Benvenga, S. Chronic lymphocytic thyroiditis: Could it be influenced by a petrochemical complex? Data from a cytological study in south-eastern Sicily. Eur. J. Endocrinol. 2015, 172, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Prummel, M.F.; Strieder, T.; Wiersinga, W.M. The environment and autoimmune thyroid diseases. Eur. J. Endocrinol. 2004, 150, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Zak, T.; Noczynska, A.; Wasikowa, R.; Zaleska-Dorobisz, U.; Golenko, A. Chronic autoimmune thyroid disease in children and adolescents in the years 1999–2004 in Lower Silesia, Poland. Hormones 2005, 4, 45–48. [Google Scholar] [PubMed]
- Langer, P.; Tajtáková, M.; Fodor, G.; Kocan, A.; Bohov, P.; Michálek, J.; Kreze, A. Increased thyroid volume and prevalence of thyroid disorders in an area heavily polluted by polychlorinated biphenyls. Eur. J. Endocrinol. 1998, 139, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, D.; Shan, Z.; Teng, X.; Guan, H.; Yu, X.; Fan, C.; Chong, W.; Yang, F.; Dai, H.; et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-years follow-up survey of populations with different iodine intakes. J. Clin. Endocrinol. Metab. 2008, 93, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Aminorroaya, A.; Janghorbani, M.; Amini, M.; Hovsepian, S.; Tabatabaei, A.; Fallahm, Z. The prevalence of thyroid dysfunction in an iodine-sufficient area in Iran. Arch. Iran. 2009, 12, 262–270. [Google Scholar]
- Rosario, P.W.; Bessa, B.; Valadao, M.M.; Purisch, S. Natural history of mild subclinical hypothyroidism: Prognostic value of ultrasound. Thyroid 2009, 19, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Vitti, P.; Cetani, F.; Catalano, F.; Concetti, R.; Pinchera, A. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J. Clin. Endocrinol. Metab. 1991, 72, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Staub, J.J.; Meier, C.; Mitrache, C.; Guglielmetti, M.; Huber, P.; Braverman, L.E. Prospective study of the spontaneous course of subclinical hypothyroidism: Prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J. Clin. Endocrinol. Metab. 2002, 87, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, J.A.; Kasamatsu, T.S.; Matsumura, L.K.; Maciel, R.M.B. Parity is not related to autoimmune thyroid disease in a population-based study of Japanese-Brazilian. Thyroid 2010, 20, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, J.A.; Matsumura, L.K.; Kasamatsu, T.S.; Ferreira, S.R.; Maciel, R.M.B. Subclinical thryroid dysfunctions are independent risk factors for mortality in a 7.5-year follow-up: The Japanese-Brazilian thyroid study. Eur. J. Endocrinol. 2010, 162, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, J.A.; Maciel, R.M.B. Pathogenesis of autoimmune thyroid diseases. Braz. Arch. Endocrinol. Metab. 2009, 53, 5–14. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J.; Tunbridge, W.M.G. The effects of drugs on endocrine function. Clin. Endocrinol. 1993, 39, 389–397. [Google Scholar] [CrossRef]
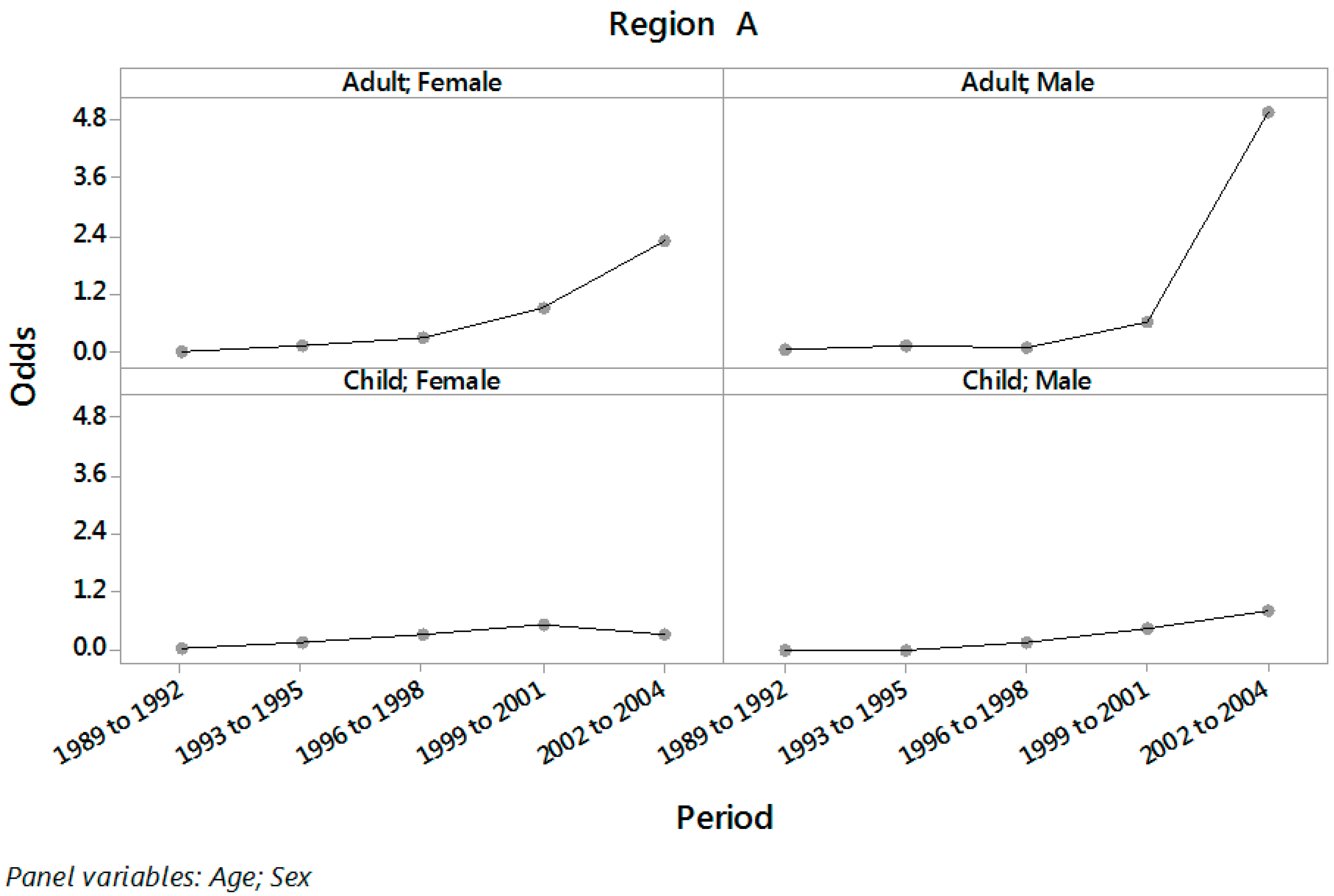
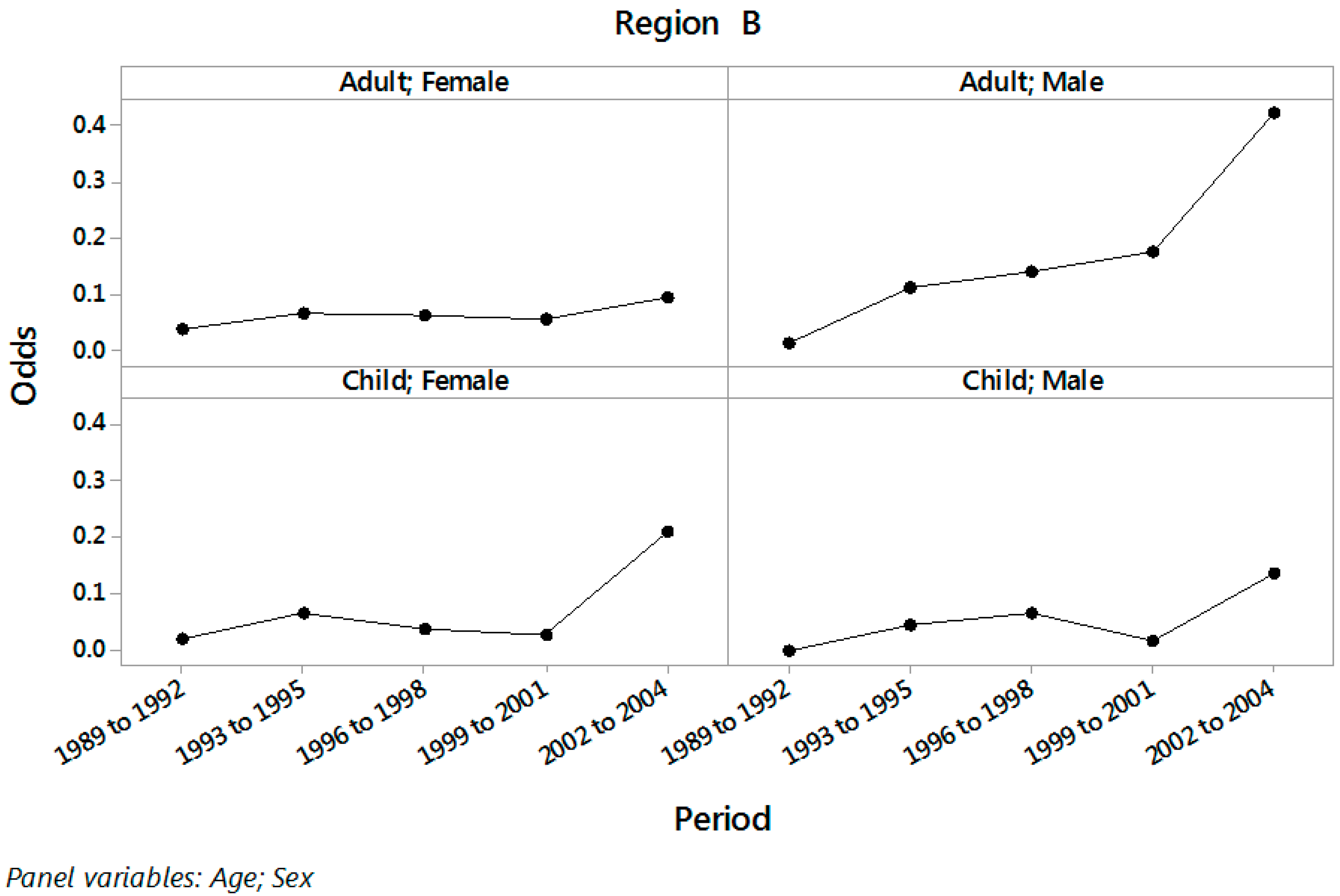
| Period | Age | Sex | Region A | Region B | ||
|---|---|---|---|---|---|---|
| Number of Patients | % of Patients with PH | Number of Patients | % of Patients with PH | |||
| 1989 to 1992 | Adult | Male | 83 | 2.4 | 75 | 1.3 |
| Female | 572 | 0.5 | 550 | 3.6 | ||
| Child | Male | 43 | 0.0 | 62 | 0.0 | |
| Female | 85 | 2.4 | 107 | 1.9 | ||
| 1993 to 1995 | Adult | Male | 58 | 8.6 | 49 | 10.2 |
| Female | 466 | 8.8 | 411 | 6.1 | ||
| Child | Male | 21 | 0.0 | 45 | 4.4 | |
| Female | 57 | 12.3 | 98 | 6.1 | ||
| 1996 to 1998 | Adult | Male | 116 | 6.0 | 65 | 12.3 |
| Female | 454 | 22.2 | 430 | 6.0 | ||
| Child | Male | 28 | 14.3 | 48 | 6.3 | |
| Female | 54 | 24.1 | 81 | 3.7 | ||
| 1999 to 2001 | Adult | Male | 71 | 38.0 | 54 | 14.8 |
| Female | 417 | 47.5 | 399 | 5.3 | ||
| Child | Male | 37 | 29.7 | 64 | 1.6 | |
| Female | 43 | 34.9 | 71 | 2.8 | ||
| 2002 to 2004 | Adult | Male | 149 | 83.2 | 27 | 29.6 |
| Female | 429 | 69.5 | 241 | 8.7 | ||
| Child | Male | 29 | 44.8 | 33 | 12.1 | |
| Female | 144 | 23.6 | 40 | 17.5 | ||
| Period | Age | Sex | Region A | Region B | ||
|---|---|---|---|---|---|---|
| Odds | CI | Odds | CI | |||
| 1989 to 2001 | Child | Female | 0.155 | (0.098; 0.245) | 0.034 | (0.019; 0.063) |
| Male | 0.066 | (0.024; 0.188) | 0.025 | (0.010; 0.066) | ||
| Adult | Female | 0.107 | (0.079; 0.145) | 0.054 | (0.044; 0.067) | |
| Male | 0.098 | (0.061; 0.158) | 0.078 | (0.043; 0.143) | ||
| 2002 to 2004 | Child | Female | 0.309 | (0.210; 0.454) | 0.212 | (0.094; 0.480) |
| Male | 0.813 | (0.391; 1.689) | 0.138 | (0.048; 0.392) | ||
| Adult | Female | 2.275 | (1.852; 2.794) | 0.095 | (0.061; 0.149) | |
| Male | 4.960 | (3.227; 7.623) | 0.421 | (0.184; 0.962) | ||
| Age | Sex | Region A | Region B | ||||
|---|---|---|---|---|---|---|---|
| Odds Ratio | CI | p | Odds Ratio | CI | p | ||
| Child | Female | 2.00 | (1.10; 3.64) | 0.024 | 6.18 | (2.23; 17.12) | <0.001 |
| Male | 12.22 | (3.43; 43.55) | <0.001 | 5.49 | (1.32; 22.86) | 0.019 | |
| Adult | Female | 21.24 | (14.72; 30.65) | <0.001 | 1.76 | (1.07; 2.88) | 0.026 |
| Male | 50.67 | (26.69; 96.21) | <0.001 | 5.38 | (1.93; 14.98) | 0.001 | |
| Age | Sex | 1989 to 2001 | 2002 to 2004 | ||||
|---|---|---|---|---|---|---|---|
| Odds Ratio | CI | p | Odds Ratio | CI | p | ||
| Child | Female | 4.54 | (2.10; 9.66) | <0.001 | 1.46 | (0.59; 3.59) | 0.413 |
| Male | 2.65 | (0.64; 10.96) | 0.179 | 5.89 | (1.64; 21.10) | 0.007 | |
| Adult | Female | 1.97 | (1.36; 2.85) | <0.001 | 23.83 | (14.56; 39.00) | <0.001 |
| Male | 1.25 | (0.58; 2.70) | 0.570 | 11.78 | (4.64; 29.89) | <0.001 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccarelli-Marino, M.A.; Saldiva André, C.D.; Singer, J.M. Overt Primary Hypothyroidism in an Industrial Area in São Paulo, Brazil: The Impact of Public Disclosure. Int. J. Environ. Res. Public Health 2016, 13, 1161. https://doi.org/10.3390/ijerph13111161
Zaccarelli-Marino MA, Saldiva André CD, Singer JM. Overt Primary Hypothyroidism in an Industrial Area in São Paulo, Brazil: The Impact of Public Disclosure. International Journal of Environmental Research and Public Health. 2016; 13(11):1161. https://doi.org/10.3390/ijerph13111161
Chicago/Turabian StyleZaccarelli-Marino, Maria Angela, Carmen Diva Saldiva André, and Julio M. Singer. 2016. "Overt Primary Hypothyroidism in an Industrial Area in São Paulo, Brazil: The Impact of Public Disclosure" International Journal of Environmental Research and Public Health 13, no. 11: 1161. https://doi.org/10.3390/ijerph13111161
APA StyleZaccarelli-Marino, M. A., Saldiva André, C. D., & Singer, J. M. (2016). Overt Primary Hypothyroidism in an Industrial Area in São Paulo, Brazil: The Impact of Public Disclosure. International Journal of Environmental Research and Public Health, 13(11), 1161. https://doi.org/10.3390/ijerph13111161






