Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Species and Culture Conditions
2.2. Removal of Bacteria from Algal Cultures
2.3. NP Treatments
2.4. Growth Analysis
2.5. Determination of Residual NP
2.5.1. NP Concentration Dissolved in the Medium
2.5.2. NP Absorbed onto Cell Surface
2.5.3. NP Concentration Absorbed into Cells
2.5.4. Determination of NP
2.6. Statistical Analysis
3. Results
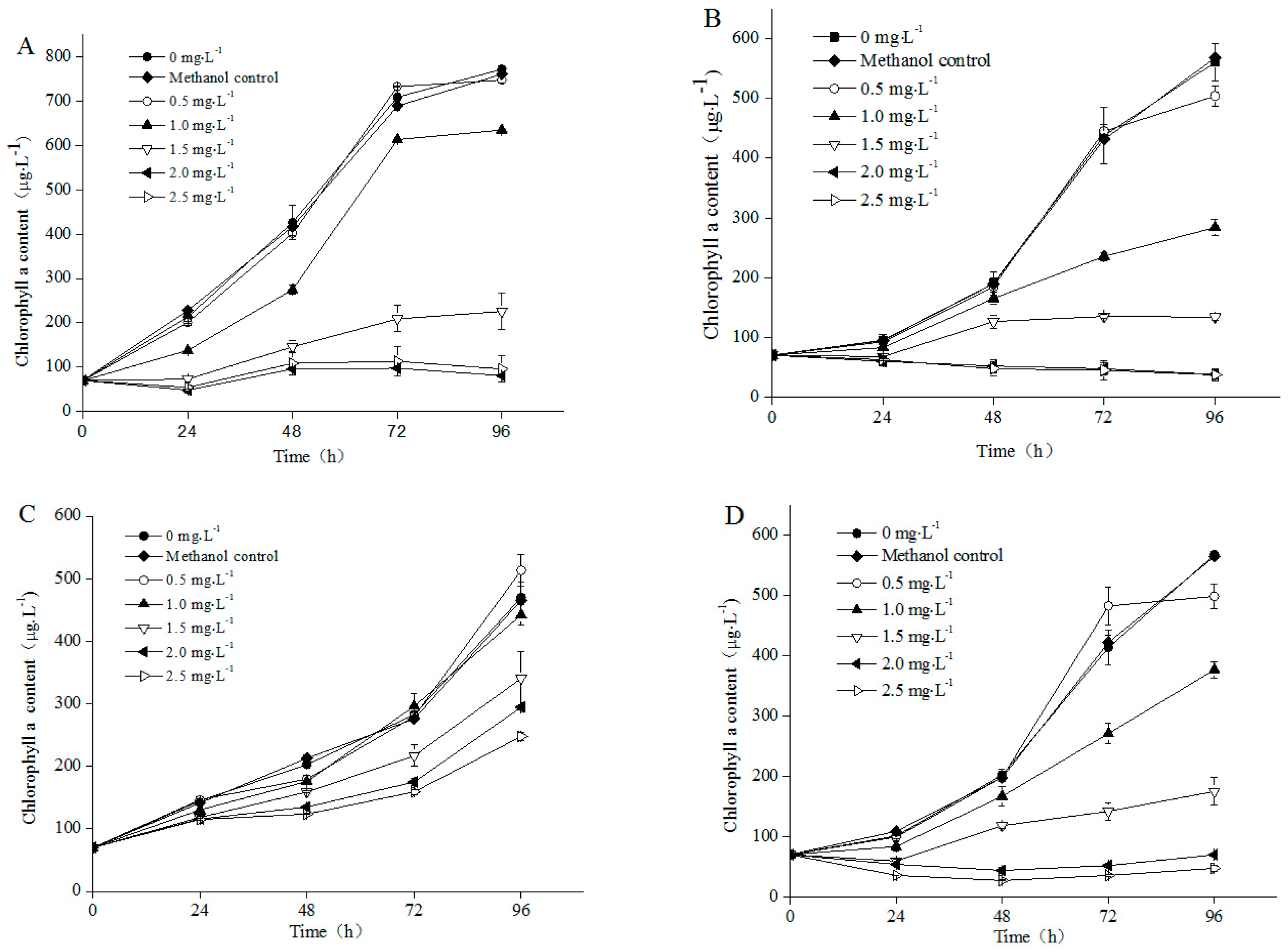
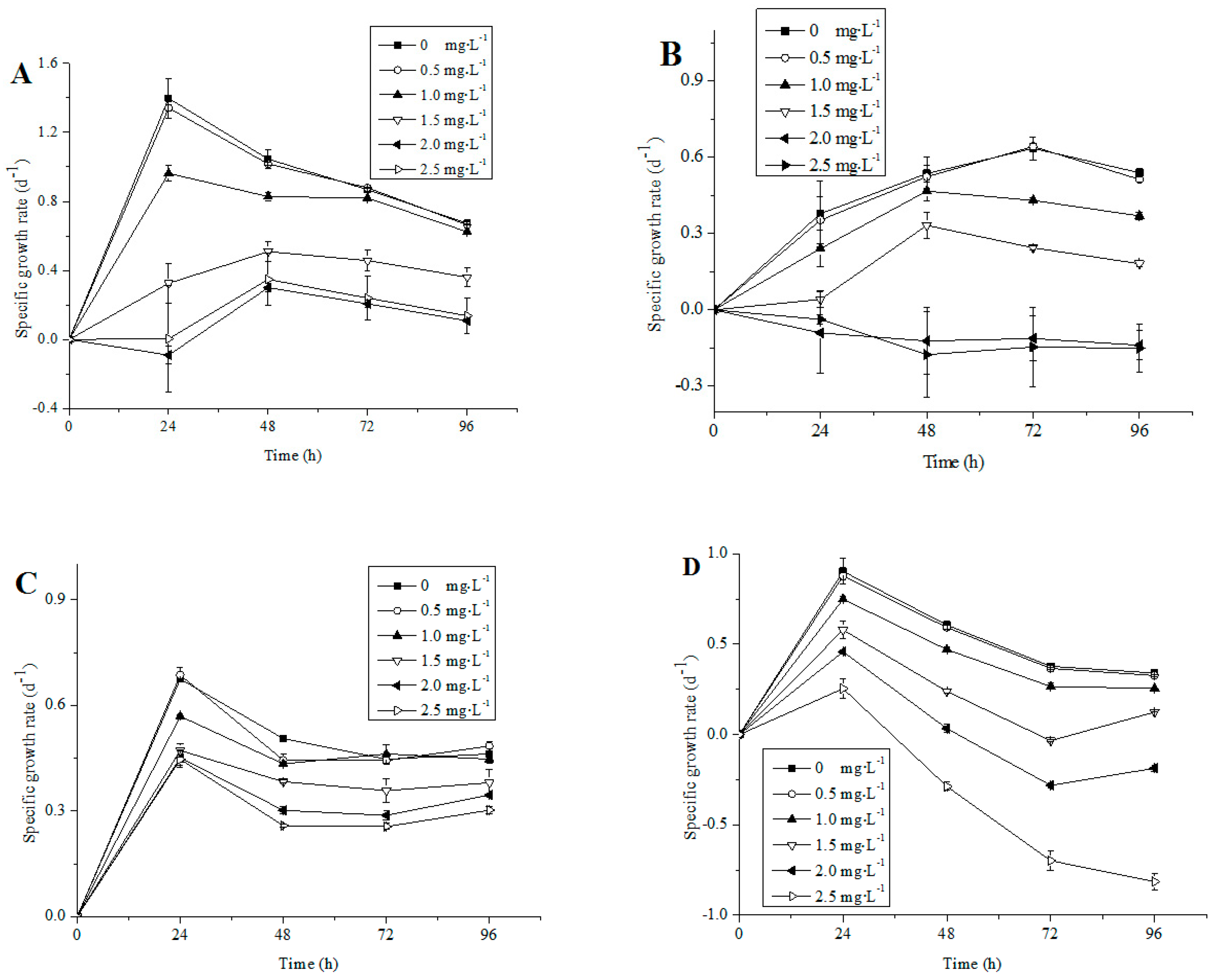
3.1. Growth of Different Microalgae Species Exposed to NP
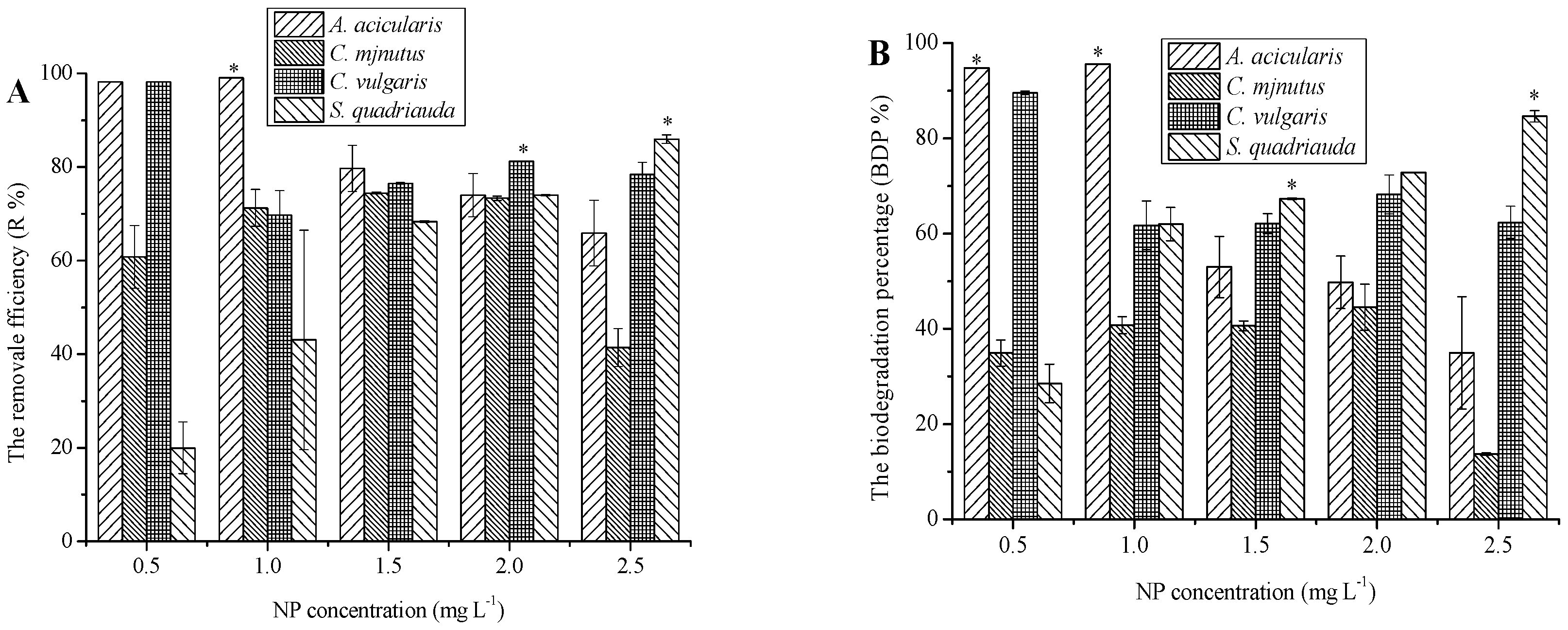
3.2. Removal of NP by Different Microalgae Species and Its Mechanisms
4. Discussion
4.1. Influence of NP on Algal Growth
4.2. Capacity of Algae for the Removal of Contaminants
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Tubau, I.; Vázquez-Suñé, E.; Carrera, J.; González, S.; Petrovic, M.; Alda, M.J.L.D.; Barceló, D. Occurrence and fate of alkylphenol polyethoxylate degradation products and linear alkylbenzene sulfonate surfactants in urban ground water: Barcelona case study. J. Hydrol. 2010, 383, 102–110. [Google Scholar] [CrossRef]
- Fan, Z.; Hu, J.; An, W.; Yang, M. Detection and occurrence of chlorinated byproducts of bisphenol A, nonylphenol, and estrogens in drinking water of China: Comparison to the parent compounds. Environ. Sci. Technol. 2013, 47, 10841–10850. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Nishiyama, H.; Nakashima, A.; Takada, H. Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: Their association with aquatic particles and sedimentary distributions. Environ. Sci. Technol. 2001, 35, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Gross-Sorokin, M.Y.; Grist, E.P.; Cooke, M.; Crane, M. Uptake and depuration of 4-nonylphenol by the benthic invertebrate Gammarus pulex: How important is feeding rate? Environ. Sci. Technol. 2003, 37, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Ahel, M.; McEvoy, J.; Giger, W. Bioaccumulation of the lipophilic metabolites of nonionic surfactants in freshwater organisms. Environ. Pollut. 1993, 79, 243–248. [Google Scholar] [CrossRef]
- Bertanza, G.; Pedrazzani, R.; Zambarda, V.; Dal Grande, M.; Icarelli, F.; Baldassarre, L. Removal of endocrine disrupting compounds from wastewater treatment plant effluents by means of advanced oxidation. Water Sci. Technol. 2010, 61, 1663. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Cain, R.B.; Schmidt, S. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 1999, 170, 291–300. [Google Scholar] [CrossRef]
- Wang, J.; Xie, P.; Guo, N. Effects of nonylphenol on the growth and microcystin production of Microcystis strains. Environ. Res. 2007, 103, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Stoichev, T.; Basto, M.C.P.; Vasconcelos, V.M.; Vasconcelos, M.T.S.D. Fate and effects of octylphenol in a Microcystis aeruginosa culture medium. Aquat. Toxicol. 2009, 92, 59–64. [Google Scholar] [PubMed]
- Elmasry, M.A.A.; Gaber, A.; Khater, E.M.H. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar]
- Fierro, S.; del Pilar Sánchez-Saavedra, M.; Copalcua, C. Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour. Technol. 2008, 99, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Jácome-Pilco, C.R.; Cristiani-Urbina, E.; Flores-Cotera, L.B.; Velasco-García, R.; Ponce-Noyola, T.; Cañizares-Villanueva, R.O. Continuous Cr (VI) removal by Scenedesmus incrassatulus in an airlift photobioreactor. Bioresour. Technol. 2009, 100, 2388–2391. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y. Selenium accumulation in unicellular green alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Baglieri, A.; Sidella, S.; Barone, V.; Fragalà, F.; Silkina, A.; Negre, M.; Gennari, M. Cultivating Chlorella vulgaris and Scenedesmus quadricauda microalgae to degrade inorganic compounds and pesticides in water. Environ. Sci. Pollut. Res. 2016, 23, 18165–18174. [Google Scholar] [CrossRef] [PubMed]
- Della Greca, M.; Pinto, G.; Pistillo, P.; Pollio, A.; Previtera, L.; Temussi, F. Biotransformation of ethinylestradiol by microalgae. Chemosphere 2008, 70, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-J.; Peng, F.-Q.; Yang, B.; Ying, G.-G. Cellular responses and bioremoval of nonylphenol and octylphenol in the freshwater green microalga Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2013, 87, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Green, D.H.; Llewellyn, L.E.; Negri, A.P.; Blackburn, S.I.; Bolch, C.J. Phylogenetic and functional diversity of the cultivable bacterial community associated with the paralytic shellfish poisoning dinoflagellate Gymnodinium catenatum. FEMS Microbiol. Ecol. 2004, 47, 345–357. [Google Scholar] [CrossRef]
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an important environment for bacteria—Phylogenetic relationships among bacterial species isolated from algae. Phycologia 2013, 52, 14–24. [Google Scholar] [CrossRef]
- Munoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Espejo, R.T. Effect of associated bacteria on the growth and toxicity of Alexandrium catenella. Appl. Environ. Microbiol. 2003, 69, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, L.; Liang, S.; Han, X.; Zhu, C.; Li, Y. Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae 2005, 4, 433–443. [Google Scholar] [CrossRef]
- Mayali, X.; Doucette, G.J. Microbial community interactions and population dynamics of an algicidal bacterium active against Karenia brevis (Dinophyceae). Harmful Algae 2002, 1, 277–293. [Google Scholar] [CrossRef]
- Hold, G.L.; Smith, E.A.; Birkbeck, T.H.; Gallacher, S. Comparison of paralytic shellfish toxin (PST) production by the dinoflagellates Alexandrium lusitanicum NEPCC 253 and Alexandrium tamarense NEPCC 407 in the presence and absence of bacteria. FEMS Microbiol. Ecol. 2001, 36, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.M.Y.; Wong, Y.S.; Tam, N.F.Y. Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere 2000, 41, 251–257. [Google Scholar] [CrossRef]
- Ibrahim, M.B.M.; Gamila, H.A. Algal bioassay for evaluating the role of algae in bioremediation of crude oil: II freshwater phytoplankton assemblages. Bull. Environ. Contam. Toxicol. 2004, 73, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.D.; Nagarkar, S.; Peart, M.R. Cyanobacterial crust in Hong Kong and comments on future research. Algae 2000, 15, 65–71. [Google Scholar]
- Lei, A.P.; Hu, Z.L.; Wong, Y.S.; Tam, N.F.Y. Removal of fluoranthene and pyrene by different microalgal species. Bioresour. Technol. 2007, 98, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Su, J.; Yang, X.; Zheng, T.; Hong, H. An efficient method to obtain axenic cultures of Alexandrium tamarense—A PSP-producing dinoflagellate. J. Microbiol. Methods 2007, 69, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-J.; Peng, F.-Q.; Zhang, L.-J.; Ying, G.-G. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2012, 19, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Correa-Reyes, G.; Viana, M.T.; Marquez-Rocha, F.J.; Licea, A.F.; Ponce, E.; Vazquez-Duhalt, R. Nonylphenol algal bioaccumulation and its effect through the trophic chain. Chemosphere 2007, 68, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, Y.; Gao, Q.; Tam, N.F.Y.; Zhu, W. Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 2010, 80, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Graff, L.; Isnard, P.; Cellier, P.; Bastide, J.; Cambon, J.P.; Narbonne, J.F.; Budzinski, H.; Vasseur, P. Toxicity of chemicals to microalgae in river and in standard waters. Environ. Toxicol. Chem. 2003, 22, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.T.; Tam, N.F.Y. Growth, photosynthesis and antioxidant responses of two microalgal species, Chlorella vulgaris and Selenastrum capricornutum, to nonylphenol stress. Chemosphere 2011, 82, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, P. Antioxidant enzyme activities of Microcystis aeruginosa in response to nonylphenols and degradation of nonylphenols by M. aeruginosa. Environ. Geochem. Health 2007, 29, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wong, Y.S.; Tam, N. Removal and biodegradation of nonylphenol by different Chlorella species. Mar. Pollut. Bull. 2011, 63, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wong, Y.; Tam, N. Removal and biodegradation of nonylphenol by immobilized Chlorella vulgaris. Bioresour. Technol. 2011, 102, 10230–10238. [Google Scholar] [CrossRef] [PubMed]
- Gattullo, C.E.; Bährs, H.; Steinberg, C.E.; Loffredo, E. Removal of bisphenol A by the freshwater green alga Monoraphidium braunii and the role of natural organic matter. Sci. Total Environ. 2012, 416, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, T.; Nagase, H.; Uchida, K.; Hiroshige, Y.; Ehara, Y.; Nishikawa, J.I.; Nishihara, T.; Miyamoto, K.; Hirata, Z. Biodegradation of bisphenol A and disappearance of its estrogenic activity by the green alga Chlorella fusca var. vacuolata. Environ. Toxicol. Chem. 2005, 24, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, G.-Z.; Tam, N.F.Y.; Luan, T.-G.; Shin, P.K.; Cheung, S.G.; Liu, Y. Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotoxicol. Environ. Saf. 2009, 72, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Lau, P.; Tam, N.; Wong, Y. Biodegradation capacity of tributyltin by two Chlorella species. Environ. Pollut. 1999, 105, 289–297. [Google Scholar] [CrossRef]
- Andrade, L.R.; Leal, R.N.; Noseda, M.; Duarte, M.E.R.; Pereira, M.S.; Mourão, P.A.S.; Farina, M.; Filho, G.M.A. Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Mar. Pollut. Bull. 2010, 60, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.N.; Luan, T.; Wong, M.H.; Tam, N.F.Y. Removal and biodegradation of polycyclic aromatic hydrocarbons by Selenastrum capricornutum. Environ. Toxicol. Chem. 2006, 25, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Teramoto, T.; Kasai, F.; Sano, T.; Tamaoki, M.; Aono, M.; Kubo, A.; Kamada, H.; Azumi, Y.; Saji, H. Glycosylation of bisphenol A by freshwater microalgae. Chemosphere 2007, 69, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hoagland, K.D.; Siegfried, B.D. Uptake and bioconcentration of atrazine by selected freshwater algae. Environ. Toxicol. Chem. 1998, 17, 1085–1090. [Google Scholar] [CrossRef]
- Tsezos, M.; Bell, J. Comparison of the biosorption and desorption of hazardous organic pollutants by live and dead biomass. Water Res. 1989, 23, 561–568. [Google Scholar] [CrossRef]
- Ji, M.K.; Kabra, A.N.; Choi, J.; Hwang, J.H.; Kim, J.R.; Abou-Shanab, R.A.I.; Oh, Y.K.; Jeon, B.H. Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol. Eng. 2014, 73, 260–269. [Google Scholar] [CrossRef]
- Babu, B.; Wu, J.-T. Production of phthalate esters by nuisance freshwater algae and cyanobacteria. Sci. Total Environ. 2010, 408, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.E.; Wu, F.; Deng, N. Photodegradation of bisphenol A in simulated lake water containing algae, humic acid and ferric ions. Environ. Pollut. 2006, 144, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, X.; Wei, J. Toxicity of the xenoestrogen nonylphenol and its biodegradation by the alga Cyclotella caspia. J. Environ. Sci. 2013, 25, 1662–1671. [Google Scholar] [CrossRef]
- Mann, R.M.; Boddy, M.R. Biodegradation of a nonylphenol ethoxylate by the autochthonous microflora in lake water with observations on the influence of light. Chemosphere 2000, 41, 1361–1369. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Petroutsos, D.; Wang, J.; Katapodis, P.; Kekos, D.; Sommerfeld, M.; Hu, Q. Toxicity and metabolism of p-chlorophenol in the marine microalga Tetraselmis marina. Aquat. Toxicol. 2007, 85, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, R.S.; Kong, R.Y. Biodegradation and enzymatic responses in the marine diatom Skeletonema costatum upon exposure to 2,4-dichlorophenol. Aquat. Toxicol. 2002, 59, 191–200. [Google Scholar] [CrossRef]
| Treatment (mg·L−1) | Microalgal Species | Amount of NP | Extra/Intra Ratio * | ||
|---|---|---|---|---|---|
| Dissolved NP (μg·L−1) | Extracellular NP (10−8 μg·cell−1) | Intracellular NP (10−8 μg·cell−1) | |||
| 0.5 | Control | 445.0 ± 10.0 | - | - | - |
| A. acicularis | 162 ± 0 | 0.9 ± 0.1 | 3.5 ± 0 | 0.26 | |
| C. minutus | 343.7 ± 1.2 | 1.0 ± 0.1 | 4.8 ± 0.7 | 0.21 | |
| C. vulgaris | 388.3 ± 11.7 | 0.8 ± 0.0 | 6.7 ± 0.6 | 0.12 | |
| S. quadriauda | 415.7 ± 32.0 | 9.7 ± 0.1 | 20.2 ± 2.8 | 0.49 | |
| 1 | Control | 941.1 ± 7.2 | - | - | - |
| A. acicularis | 405.9 ± 15.6 | 3.0 ± 0 | 14.1 ± 0.6 | 0.21 | |
| C. minutus | 515.9 ± 62.1 | 2.0 ± 0.2 | 14.5 ± 3.7 | 0.15 | |
| C. vulgaris | 723.7 ± 13.7 | 2.7 ± 0.1 | 9.6 ± 0.6 | 0.28 | |
| S. quadriauda | 770.2 ± 5.2 | 14.1 ± 3.2 | 37.2 ± 2.5 | 0.85 | |
| 1.5 | Control | 1479.7 ± 10.2 | - | - | - |
| A. acicularis | 665.3 ± 87.8 | 8.4 ± 0.8 | 37.0 ± 2.4 | 0.23 | |
| C. minutus | 964.9 ± 22.4 | 3.7 ± 0.6 | 24.8 ± 0.8 | 0.15 | |
| C. vulgaris | 1464.3 ± 154.8 | 8.1 ± 0.3 | 23.7 ± 2.9 | 0.35 | |
| S. quadriauda | 1477.8 ± 16.4 | 18.2 ± 0.6 | 61.7 ± 6.5 | 0.3 | |
| 2 | Control | 1890 ± 13.0 | - | - | - |
| A. acicularis | 845.6 ± 0.3 | 10.4 ± 0.2 | 49.8 ± 1.1 | 0.21 | |
| C. minutus | 1236.6 ± 41.6 | 4.9 ± 0.5 | 28.8 ± 2.6 | 0.17 | |
| C. vulgaris | 1305.2 ± 211.6 | 10.2 ± 1.0 | 33.4 ± 3.9 | 0.31 | |
| S. quadriauda | 1795.6 ± 132.1 | 23.2 ± 2.9 | 104.6 ± 0.6 | 0.22 | |
| 2.5 | Control | 2401.0 ± 92.6 | - | - | - |
| A. acicularis | 1360.5 ± 169.1 | 16.9 ± 1.2 | 77.0 ± 1.9 | 0.22 | |
| C. minutus | 1606.5 ± 136.2 | 6.6 ± 0.6 | 38.0 ± 0.5 | 0.16 | |
| C. vulgaris | 2308.5 ± 52.5 | 13.1 ± 1.4 | 38.3 ± 3.3 | 0.34 | |
| S. quadriauda | 1911.0 ± 8.7 | 50.3 ± 0.3 | 124.4 ± 1.3 | 0.4 | |
| Treatment (mg·L−1) | Microalgal Species | Amount of NP | Extra/Intra Ratio * | ||
|---|---|---|---|---|---|
| Dissolved NP (μg·L−1) | Extracellular NP (10−8 μg·cell−1) | Intracellular NP (10−8 μg·cell−1) | |||
| 0.5 | Control | 448.3 ± 5.6 | - | - | - |
| A. acicularis | 9.1 ± 0 | 0.2 ± 0 | 0.5 ± 0.1 | 0.33 | |
| C. minutus | 191.0 ± 0 | 0.1 ± 0 | 2.3 ± 0.3 | 0.06 | |
| C. vulgaris | 270.8 ± 3.4 | 1.0 ± 0.1 | 3.8 ± 0.6 | 0.26 | |
| S. quadriauda | 323.0 ± 0.9 | 1.7 ± 0.1 | 2.1 ± 0.2 | 0.85 | |
| 1 | Control | 940.2 ± 10.1 | - | - | - |
| A. acicularis | 117.0 ± 5.7 | 0.6 ± 0.1 | 1.9 ± 0.2 | 0.3 | |
| C. minutus | 402.4 ± 19.5 | 1.2 ± 0.1 | 7.1 ± 1.9 | 0.18 | |
| C. vulgaris | 549.1 ± 8.6 | 2.3 ± 0.2 | 7.8 ± 0.7 | 0.3 | |
| S. quadriauda | 478.1 ± 7.2 | 0.6 ± 0 | 2.7 ± 0.4 | 0.23 | |
| 1.5 | Control | 1478.9 ± 23.6 | - | - | - |
| A. acicularis | 539.3 ± 7.8 | 4.6 ± 0.7 | 19.2 ± 5.4 | 0.24 | |
| C. minutus | 760.1 ± 13.7 | 2.7 ± 0.1 | 16.5 ± 0.4 | 0.16 | |
| C. vulgaris | 840.6 ± 67.1 | 4.2 ± 0.4 | 18.7 ± 1.1 | 0.23 | |
| S. quadriauda | 720.2 ± 14.4 | 0.9 ± 0 | 4.0 ± 1.0 | 0.26 | |
| 2 | Control | 1873.2 ± 26.0 | - | - | - |
| A. acicularis | 599.3 ± 1.3 | 7.1 ± 0.2 | 24.1 ± 0.2 | 0.29 | |
| C. minutus | 1006.7 ± 29.1 | 3.6 ± 0.1 | 19.0 ± 2.1 | 0.18 | |
| C. vulgaris | 863.2 ± 45.2 | 5.5 ± 1.0 | 24.1 ± 4.1 | 0.23 | |
| S. quadriauda | 944.6 ± 21.6 | 1.9 ± 0.1 | 5.8 ± 0.6 | 0.32 | |
| 2.5 | Control | 2399.6 ± 27.8 | - | - | - |
| A. acicularis | 819.4 ± 31.7 | 11.3 ± 1.3 | 65.2 ± 10.0 | 0.17 | |
| C. minutus | 1429.4 ± 62.1 | 6.1 ± 0.2 | 27.7 ± 1.3 | 0.22 | |
| C. vulgaris | 1529.3 ± 46.4 | 7.2 ± 0.9 | 28.8 ± 3.1 | 0.25 | |
| S. quadriauda | 1391.2 ± 11.3 | 3.4 ± 0.5 | 12.8 ± 0.4 | 0.29 | |
| Treatment (mg·L−1) | Microalgal Species | Amount of NP | Extra/Intra Ratio * | ||
|---|---|---|---|---|---|
| Dissolved NP (μg·L−1) | Extracellular NP (10−8 μg·cell−1) | Intracellular NP (10−8 μg·cell−1) | |||
| 0.5 | Control | 448.0 ± 9.1 | - | - | - |
| A. acicularis | 9.1 ± 0 | 0.0 ± 0 | 0.4 ± 0 | 0.04 | |
| C. minutus | 196.1 ± 37.3 | 0.1 ± 0 | 1.5 ± 0.2 | 0.08 | |
| C. vulgaris | 9.1 ± 0 | 0.5 ± 0.0 | 2.6 ± 0 | 0.2 | |
| S. quadriauda | 200.0 ± 22.5 | 0.3 ± 0.0 | 0.1 ± 0 | 3.04 | |
| 1 | Control | 943.9 ± 8.5 | - | - | - |
| A. acicularis | 9.1 ± 0 | 0.2 ± 0 | 0.7 ± 0 | 0.3 | |
| C. minutus | 287.3 ± 32.4 | 0.1 ± 0 | 3.8 ± 0.9 | 0.04 | |
| C. vulgaris | 302.9 ± 42.8 | 1.7 ± 0.4 | 4.3 ± 0.9 | 0.4 | |
| S. quadriauda | 335.1 ± 19.1 | 0.4 ± 0 | 0.6 ± 0.1 | 0.6 | |
| 1.5 | Control | 1475.9 ± 14.8 | - | - | - |
| A. acicularis | 304.6 ± 60.5 | 3.9 ± 0.2 | 13.0 ± 0.2 | 0.3 | |
| C. minutus | 384.4 ± 2.2 | 0.7 ± 0.1 | 14.2 ± 0.3 | 0.05 | |
| C. vulgaris | 352.6 ± 2.3 | 3.6 ± 0.6 | 11.9 ± 2.2 | 0.31 | |
| S. quadriauda | 474.8 ± 2.7 | 0.4 ± 0 | 1.0 ± 0 | 0.42 | |
| 2 | Control | 1880.5 ± 7.7 | - | - | - |
| A. acicularis | 520.1 ± 75.9 | 4.8 ± 0.3 | 14.0 ± 0.4 | 0.34 | |
| C. minutus | 533.5 ± 7.8 | 1.1 ± 0.2 | 14.0 ± 1.2 | 0.08 | |
| C. vulgaris | 375.7 ± 0.7 | 3.1 ± 0.7 | 8.8 ± 2.6 | 0.37 | |
| S. quadriauda | 519.9 ± 1.8 | 0.7 ± 0 | 1.77 ± 0.2 | 0.4 | |
| 2.5 | Control | 2397.9 ± 77.8 | - | - | - |
| A. acicularis | 803.1 ± 102.9 | 6.5 ± 0.05 | 23.9 ± 3.6 | 0.27 | |
| C. minutus | 1465.3 ± 82.5 | 1.6 ± 0.4 | 19.6 ± 0.4 | 0.08 | |
| C. vulgaris | 539.9 ± 52.8 | 3.5 ± 0.7 | 12.6 ± 1.8 | 0.28 | |
| S. quadriauda | 351.6 ± 18.8 | 2.6 ± 0.2 | 5.7 ± 0.8 | 0.46 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, N.; Sun, X.; Zhong, Y.; Sun, K.; Liu, W.; Duan, S. Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae. Int. J. Environ. Res. Public Health 2016, 13, 1239. https://doi.org/10.3390/ijerph13121239
He N, Sun X, Zhong Y, Sun K, Liu W, Duan S. Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae. International Journal of Environmental Research and Public Health. 2016; 13(12):1239. https://doi.org/10.3390/ijerph13121239
Chicago/Turabian StyleHe, Ning, Xian Sun, Yu Zhong, Kaifeng Sun, Weijie Liu, and Shunshan Duan. 2016. "Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae" International Journal of Environmental Research and Public Health 13, no. 12: 1239. https://doi.org/10.3390/ijerph13121239






