Perfluorinated Compounds in Greenhouse and Open Agricultural Producing Areas of Three Provinces of China: Levels, Sources and Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sampling Campaign
2.3. Sample Extraction and Cleanup
2.3.1. Agricultural Products
2.3.2. Water Samples
2.3.3. Soil Samples
2.4. Instrumentation and Analysis
2.5. Quality Assurance and Quality Control
2.6. Data Analysis
3. Results and Discussion
3.1. Background Pollution of PFAAs from Instruments
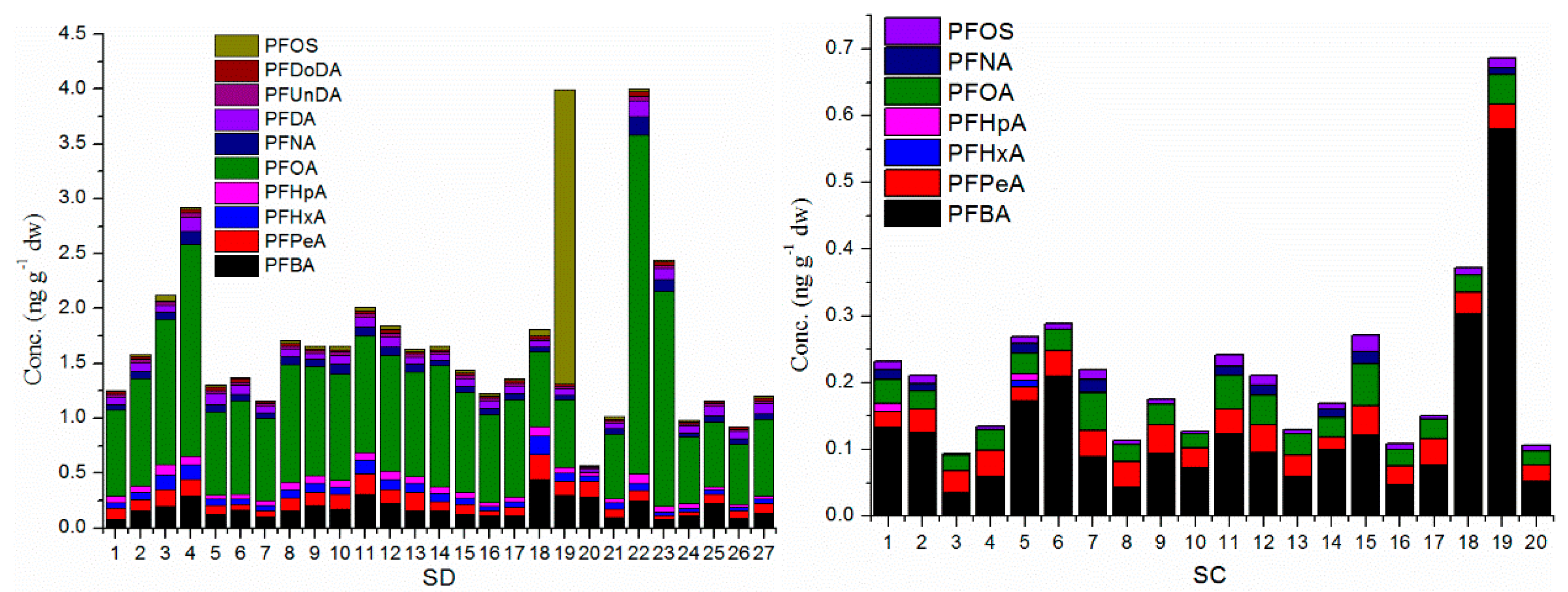
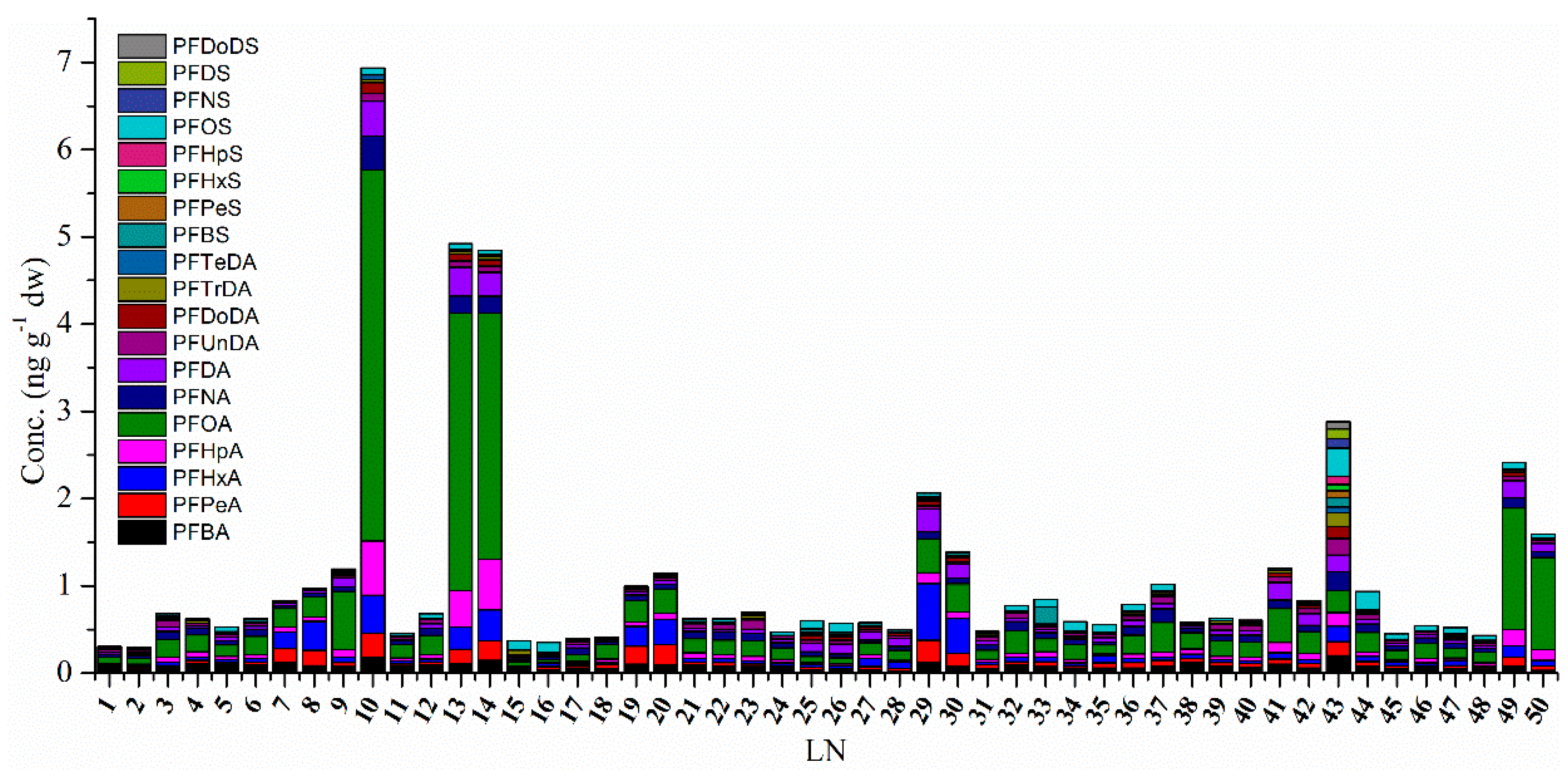
3.2. The Level of PFAAs in Soil Samples
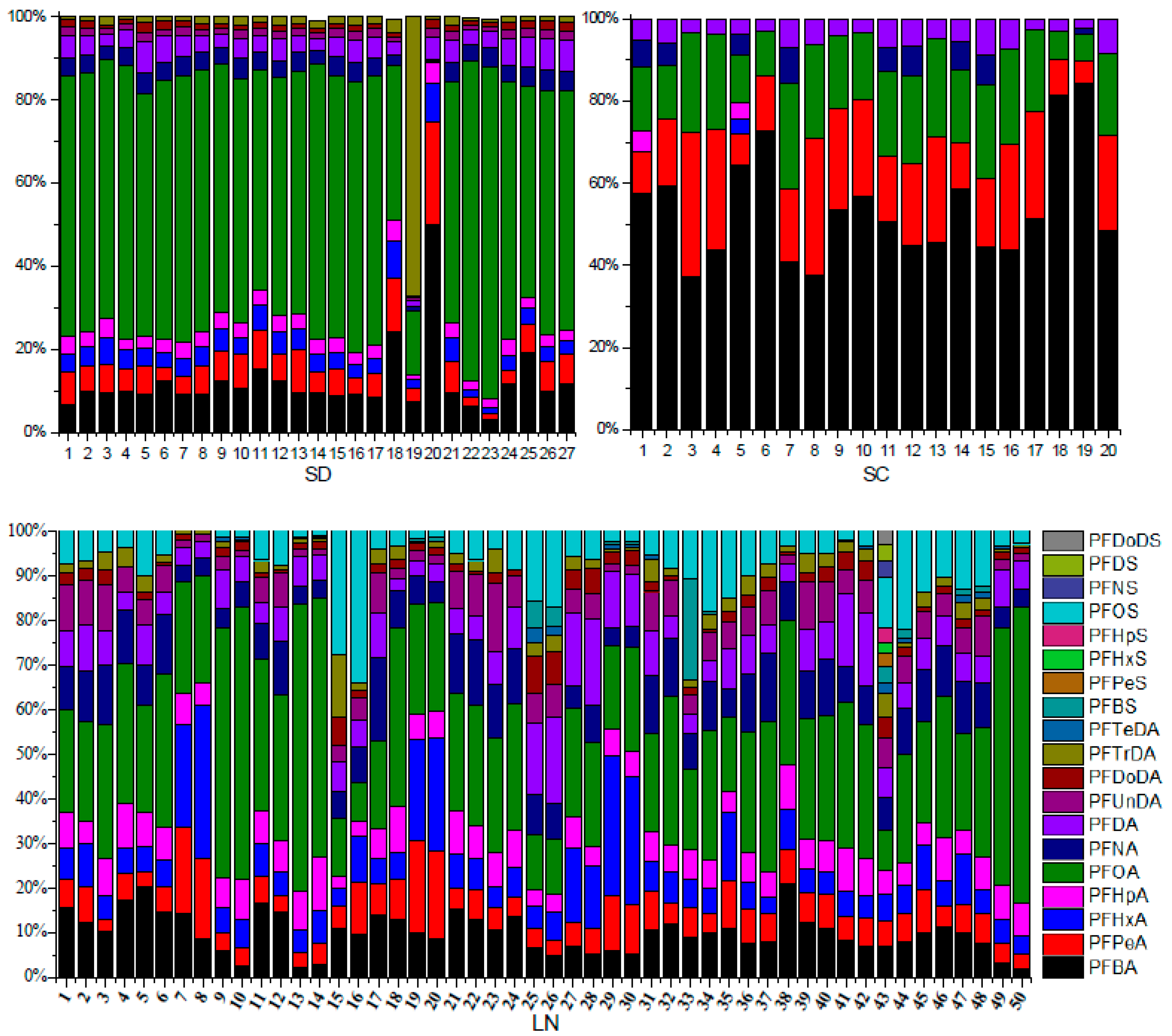
3.3. The Concentration and Contribution of PFAA Compounds in Soil Samples
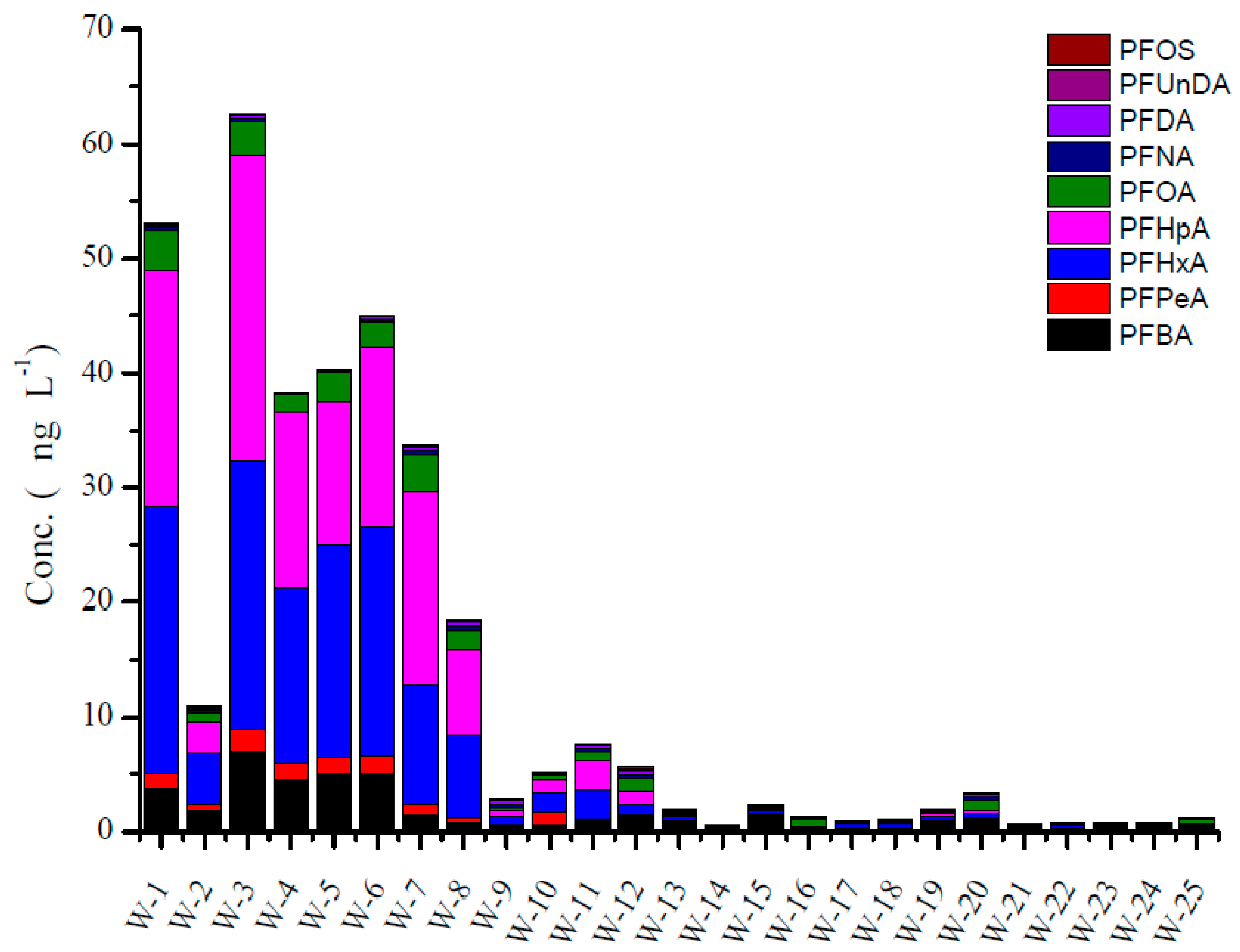
3.4. The Concentration of PFAA Compounds in Irrigation Water and Agricultural Products from LN Province
3.5. Daily Intake Evaluation of PFOA through Vegetables by the Population in Liaoning Province
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146a–152a. [Google Scholar] [CrossRef] [PubMed]
- Happonen, M.; Koivusalo, H.; Malve, O.; Perkola, N.; Juntunen, J.; Huttula, T. Contamination risk of raw drinking water caused by PFOA sources along a river reach in south-western Finland. Sci. Total Environ. 2016, 541, 74–82. [Google Scholar] [CrossRef] [PubMed]
- 3M-Company, 2000, Phase-Out Plan for POSF-Based Products. Available online: www.regulation.gov (accessed on 5 May 2016).
- Naile, J.E.; Garrison, A.W.; Avants, J.K.; Washington, J.W. Isomers/enantiomers of perfluorocarboxylic acids: Method development and detection in environmental samples. Chemosphere 2016, 144, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.J.; Li, W.W.; Lam, P.K.S.; Yu, H.Q. Boiling significantly promotes photodegradation of perfluorooctane sulfonate. Chemosphere 2015, 138, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci. Total Environ. 2015, 524, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Wang, L.; Zhang, T.; Li, F.; He, N.; Alder, A. Perfluoroalkyl compounds in municipal WWTPs in Tianjin, China—Concentrations, distribution and mass flow. Environ. Sci. Pollut. Res. 2012, 19, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Guo, L.-H. Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin. Arch. Toxicol. 2009, 83, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hundal, L.S.; Kumar, K.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environ. Sci. Technol. 2014, 48, 7858–7865. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, W.; Herzke, D.; Huber, S.; Hajslova, J.; Pulkrabova, J.; Brambilla, G.; De Filippis, S.P.; Bervoets, L.; de Voogt, P. Occurrence of perfluorinated alkylated substances in cereals, salt, sweets and fruit items collected in four European countries. Chemosphere 2015, 129, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, J.-L.; Li, H.-H.; Hu, G.-C.; Li, H.-S. Analysis of trace metals and perfluorinated compounds in 43 representative tea products from south China. J. Food Sci. 2014, 79, C1123–C1129. [Google Scholar] [CrossRef] [PubMed]
- Herzke, D.; Huber, S.; Bervoets, L.; D’Hollander, W.; Hajslova, J.; Pulkrabova, J.; Brambilla, G.; De Filippis, S.P.; Klenow, S.; Heinemeyer, G.; et al. Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ. Sci. Pollut. Res. 2013, 20, 7930–7939. [Google Scholar] [CrossRef] [PubMed]
- Noorlander, C.W.; van Leeuwen, S.P.J.; te Biesebeek, J.D.; Mengelers, M.J.B.; Zeilmaker, M.J. Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. J. Agric. Food Chem. 2011, 59, 7496–7505. [Google Scholar] [CrossRef] [PubMed]
- Klenow, S.; Heinemeyer, G.; Brambilla, G.; Dellatte, E.; Herzke, D.; de Voogt, P. Dietary exposure to selected perfluoroalkyl acids (PFAAs) in four European regions. Food Addit. Contam. 2013, 30, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Zhou, M.; Ren, X.H.; Zhang, Y.W.; Gan, Z.W.; Sun, H.W. Distribution of perfluorooctane sulfonate and perfluorooctanoic acid in different tissues of fishes and vegetables from Dahuangpu Wetland Nature Reserve, Tianjin city, China. J. Agro-Environ. Sic. Chin. 2012, 31, 2321–2327. [Google Scholar]
- Felizeter, S.; McLachlan, M.S.; De Voogt, P. Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. J. Agric. Food Chem. 2014, 62, 3334–3342. [Google Scholar] [CrossRef] [PubMed]
- Krippner, J.; Brunn, H.; Falk, S.; Georgii, S.; Schubert, S.; Stahl, T. Effects of chain length and pH on the uptake and distribution of perfluoroalkyl substances in Maize (Zea mays). Chemosphere 2014, 94, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bizkarguenaga, E.; Zabaleta, I.; Prieto, A.; Fernandez, L.A.; Zuloaga, O. Uptake of 8:2 perfluoroalkyl phosphate diester and its degradation products by carrot and lettuce from compost-amended soil. Chemosphere 2016, 152, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Halsall, C.; Codling, G.; Xie, Z.Y.; Xu, B.Q.; Zhao, Z.; Xue, Y.G.; Ebinghaus, R.; Jones, K.C. Accumulation of perfluoroalkyl compounds in Tibetan mountain snow: Temporal patterns from 1980 to 2010. Environ. Sci. Technol. 2014, 48, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Wang, T.Y.; Wang, P.; Zhang, Y.Q.; Li, Q.F.; Lu, Y.L.; Giesy, J.P. Are levels of perfluoroalkyl substances in soil related to urbanization in rapidly developing coastal areas in north China? Environ. Pollut. 2015, 199, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Lin, Y.; Wang, L.; Zhang, X.; Liu, Y.; Geng, X.; Zhao, L.; Li, F.; Kannan, K. Perfluorinated compounds in human blood, water, edible freshwater fish, and seafood in China: Daily intake and regional differences in human exposures. J. Agric. Food Chem. 2011, 59, 11168–11176. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Lu, Y.L.; Wang, T.Y.; Liu, S.J.; Jones, K.; Sweetman, A. Estimation of PFOS emission from domestic sources in the eastern coastal region of China. Environ. Int. 2013, 59, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Wang, T.Y.; Liu, S.J.; Jones, K.C.; Sweetman, A.J.; Lu, Y.L. Industrial source identification and emission estimation of perfluorooctane sulfonate in Hina. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wang, T.Y.; Wang, P.; Luo, W.; Lu, Y.L.; Romesh, K.; Giesy, J. Perfluoroalkyl substances in soils around the Nepali Koshi River: Levels, distribution, and mass balance. Environ. Sci. Pollut. Res. 2014, 21, 9201–9211. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.; Woldegiorgis, A.; Norstrom, K.; Bibi, M.; Lindberg, M.; Osteras, A.H. Historical usage of aqueous film forming foam: A case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere 2015, 129, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, T.Y.; Giesy, J.P.; Lu, Y.L. Perfluorinated compounds in soils from Liaodong Bay with concentrated fluorine industry parks in China. Chemosphere 2013, 91, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Oliaei, F.; Kriens, D.; Weber, R.; Watson, A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ. Sci. Pollut. Res. 2013, 20, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, M.; Zhang, T.; Sun, H. Polyfluorinated and perfluorinated chemicals in precipitation and runoff from cities across eastern and central China. Arch. Environ. Contam. Toxicol. 2013, 64, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, H.; Li, B.; Hu, H.; Zhang, T.; Yamazaki, E.; Taniyasu, S.; Yamashita, N.; Sun, H. Distribution and primary source analysis of per-and poly-fluoroalkyl substances with different chain lengths in surface and groundwater in two cities, north China. Ecotoxicol. Environ. Saf. 2014, 108, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Simcik, M.F.; Halbach, T.R.; Gulliver, J.S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: Migration and implications for human exposure. Water Res. 2015, 72, 64–74. [Google Scholar] [CrossRef] [PubMed]



| PFAA | Soils | Agricultural Products | Irrigation Water | |||
|---|---|---|---|---|---|---|
| Recovery (%) | RSD | Recovery (%) | RSD | Recovery (%) | RSD | |
| PFBA | 69.6 | 0.86–9.12 | 96.3 | 2.34–5.77 | 124.7 | 5.43–6.11 |
| PFPeA | 70.9 | 0.92–5.32 | 119.1 | 9.54–12.1 | 109.8 | 8.99–9.11 |
| PFHxA | 70.1 | 1.23–8.36 | 123.3 | 7.44–9.21 | 94.2 | 11.2–15.9 |
| PFHpA | 72.6 | 4.16–9.09 | 112.3 | 10.2–14.1 | 92.8 | 4.12–8.99 |
| PFOA | 76.9 | 5.62–7.88 | 92.6 | 10.1–15.2 | 86.6 | 3.56–11.2 |
| PFNA | 71.0 | 2.32–6.89 | 76.4 | 2.45–3.66 | 98.9 | 3.56–6.88 |
| PFDA | 72.2 | 1.24–12.1 | 76.1 | 5.21–10.3 | 99.1 | 11.3–16.7 |
| PFUnDA | 74.4 | 8.33–13.6 | 77.5 | 10.2–13.4 | 95.1 | 12.5–13.1 |
| PFDoDA | 70.1 | 9.23–11.6 | 71.3 | 4.21–8.99 | 72.3 | 2.34–12.1 |
| PFTrDA | 68.1 | 3.56–15.7 | 57.8 | 2.55–6.55 | 51.8 | 5.76–13.5 |
| PFTeDA | 66.2 | 12.1–15.4 | 44.8 | 4.35–8.99 | 43.1 | 12.6–15.7 |
| PFHxDA | 43.5 | - | 34.4 | - | 35.4 | - |
| PFODA | 41.1 | - | 59.4 | - | 31.3 | - |
| PFBS | 73.9 | 1.54–3.66 | 123.0 | 9.11–10.2 | 112.4 | 9.07–10.9 |
| PFPeS | 73.4 | 6.34–2.43 | 104.5 | 2.11–5.44 | 108.8 | 9.45–13.7 |
| PFHxS | 70.3 | 2.56–7.66 | 79.4 | 6.12–9.11 | 97.7 | 4.56–12.9 |
| PFHpS | 72.4 | 3.45–8.45 | 76.4 | 3.11–8.99 | 100.1 | 4.56–14.8 |
| PFOS | 70.5 | 2.67–10.1 | 80.4 | 5.66–9.32 | 109.8 | 2.45–5.87 |
| PFNS | 68.9 | 2.32–10.5 | 80.9 | 9.34–10.6 | 119.6 | 9.12–15.6 |
| PFDS | 68.3 | 4.55–8.6 | 75.1 | 2.34–9.55 | 117.7 | 10.5–15.8 |
| PFDoDS | 64.4 | - | 51.9 | - | 59.5 | - |
| Country | TDI (ng·kg−1·BW·day−1) | DI (ng·kg−1·day−1) | Ref. |
|---|---|---|---|
| USA | 20 | 1200 | USA EPA |
| Japan | 5.4 | 324 | Japan EPA |
| Australia | 1500 | 90,000 | Australia EPA |
| EFSA | 1500 | 90,000 | EFSA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tan, D.; Geng, Y.; Wang, L.; Peng, Y.; He, Z.; Xu, Y.; Liu, X. Perfluorinated Compounds in Greenhouse and Open Agricultural Producing Areas of Three Provinces of China: Levels, Sources and Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 1224. https://doi.org/10.3390/ijerph13121224
Zhang Y, Tan D, Geng Y, Wang L, Peng Y, He Z, Xu Y, Liu X. Perfluorinated Compounds in Greenhouse and Open Agricultural Producing Areas of Three Provinces of China: Levels, Sources and Risk Assessment. International Journal of Environmental Research and Public Health. 2016; 13(12):1224. https://doi.org/10.3390/ijerph13121224
Chicago/Turabian StyleZhang, Yanwei, Dongfei Tan, Yue Geng, Lu Wang, Yi Peng, Zeying He, Yaping Xu, and Xiaowei Liu. 2016. "Perfluorinated Compounds in Greenhouse and Open Agricultural Producing Areas of Three Provinces of China: Levels, Sources and Risk Assessment" International Journal of Environmental Research and Public Health 13, no. 12: 1224. https://doi.org/10.3390/ijerph13121224





