Quantitative Detection of Trace Malachite Green in Aquiculture Water Samples by Extractive Electrospray Ionization Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
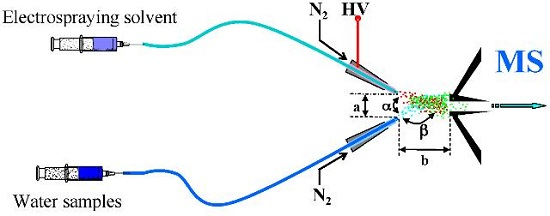
2.2. EESI-MS Condition
2.3. Preparation of Spiked Samples
3. Results and Discussion
3.1. Detection of Malachite Green by EESI-MS
3.2. Optimization of EESI Parameters
3.2.1. Electrospray Voltage
3.2.2. Sample Flow Rate
3.2.3. Temperature of the Heated Capillary
3.2.4. Sheath Gas Pressure
3.3. Quantification of Malachite Green in Lake Water
3.4. Real-Life Sample Analyses
3.5. Sample Consumption and Analysis Speed
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [PubMed]
- Mitrowska, K.; Posyniak, A.; Zmudzki, J. Determination of malachite green and leucomalachite green residues in water using liquid chromatography with visible and fluorescence detection and confirmation by tandem mass spectrometry. J. Chromatogr. A 2008, 1207, 94–100. [Google Scholar]
- Sudova, E.; Machova, J.; Svobodova, Z.; Vesely, T. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: A review. Vet. Med. 2007, 52, 527–539. [Google Scholar]
- Khodabakhshi, A.; Amin, M. Determination of malachite green in trout tissue and effluent water from fish farms. Int. J. Environ. Heal. Eng. 2012, 1, 10. [Google Scholar]
- Šafařı́k, I.; Šafařı́ková, M. Detection of low concentrations of malachite green and crystal violet in water. Water Res. 2002, 36, 196–200. [Google Scholar] [CrossRef]
- Afkhami, A.; Moosavi, R.; Madrakian, T. Preconcentration and spectrophotometric determination of low concentrations of malachite green and leuco-malachite green in water samples by high performance solid phase extraction using maghemite nanoparticles. Talanta 2010, 82, 785–789. [Google Scholar] [PubMed]
- An, L.; Deng, J.; Zhou, L.; Li, H.; Chen, F.; Wang, H.; Liu, Y. Simultaneous spectrophotometric determination of trace amount of malachite green and crystal violet in water after cloud point extraction using partial least squares regression. J. Hazard. Mater. 2010, 175, 883–888. [Google Scholar]
- Han, M.L.; Chen, Y.Y.; Kai, J.Y.; Yang, M.L. Determination of malachite green in environmental water samples by spectrophotometry with dispersive liquid-liquid microextraction. Chin. J. Spectrosc. Lab. 2011, 28, 205–209. [Google Scholar]
- Zhang, Z.; Zhou, K.; Bu, Y.; Shan, Z.; Liu, J.; Wu, X.; Yang, L.; Chen, Z. Determination of malachite green and crystal violet in environmental water using temperature-controlled ionic liquid dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Anal. Meth. 2012, 4, 429–433. [Google Scholar]
- Long, C.; Mai, Z.; Yang, Y.; Zhu, B.; Xu, X.; Lu, L.; Zou, X. Determination of multi-residue for malachite green, gentian violet and their metabolites in aquatic products by high-performance liquid chromatography coupled with molecularly imprinted solid-phase extraction. J. Chromatogr. A 2009, 1216, 2275–2281. [Google Scholar]
- Maleki, R.; Farhadi, K.; Nikkhahi, Y. Trace determination of malachite green in water samples using dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography-diode array detection. Int. J. Environ. Anal. Chem. 2012, 92, 1026–1035. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, J.D.; Lin, C.H. Optimization of the separation of malachite green in water by capillary electrophoresis Raman spectroscopy (CE-RS) based on the stacking and sweeping modes. Talanta 2007, 72, 368–372. [Google Scholar] [PubMed]
- Scherpenisse, P.; Bergwerff, A.A. Determination of residues of malachite green in finfish by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2005, 529, 173–177. [Google Scholar]
- Tao, Y.D.; Chao, X.; Yu, H.; Yuan, H.P.; Liu, Z.; Huang, L.; Wang, Y.; Yuan, Z. Simultaneous determination of malachite green, gentian violet and their leuco-metabolites in shrimp and salmon by liquid chromatography-tandem mass spectrometry with accelerated solvent extraction and auto solid-phase dean-up. Food Control 2011, 22, 1246–1252. [Google Scholar] [CrossRef]
- Stead, S.L.; Ashwin, H.; Johnston, B.H.; Dallas, A.; Kazakov, S.A.; Tarbin, J.A.; Sharman, M.; Kay, J.; Keely, B.J. An RNA-aptamer-based assay for the detection and analysis of malachite green and leucomalachite green residues in fish tissue. Anal. Chem. 2010, 82, 2652–2660. [Google Scholar]
- Li, X.; Hu, B.; Ding, J.; Chen, H. Rapid characterization of complex viscous samples at molecular levels by neutral desorption extractive electrospray ionization mass spectrometry. Nat. Protoc. 2011, 6, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, S.; Li, M.; Hu, B.; Li, J.; Wang, J. Sensitive detection of native proteins using extractive electrospray ionization mass spectrometry. Angew. Chem. Int. Ed. 2010, 122, 3117–3120. [Google Scholar]
- Chen, H.; Yang, S.; Wortmann, A.; Zenobi, R. Neutral desorption sampling of living objects for rapid analysis by extractive electrospray ionization mass spectrometry. Angew. Chem. Int. Ed. 2007, 119, 7735–7738. [Google Scholar]
- Luo, M.; Hu, B.; Zhang, X.; Peng, D.; Chen, H.; Zhang, L.; Huan, Y. Extractive electrospray ionization mass spectrometry for sensitive detection of uranyl species in natural water samples. Anal. Chem. 2010, 82, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Venter, A.; Cooks, R.G. Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation. Chem. Commun. 2006. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Zhang, X. Principle and application of ambient mass spectrometry for direct analysis of complex samples. Chin. J. Anal. Chem. 2010, 38, 1069–1088. [Google Scholar]
- Law, W.S.; Wang, R.; Hu, B.; Berchtold, C.; Meier, L.; Chen, H.; Zenobi, R. On the mechanism of extractive electrospray ionization. Anal. Chem. 2010, 82, 4494–4500. [Google Scholar] [PubMed]
- Liu, C.; Hu, B.; Shi, J.; Li, J.; Zhang, X.; Chen, H. Determination of uranium isotopic ratio (235U/238U) using extractive electrospray ionization tandem mass spectrometry. J. Anal. Atom. Spectrom. 2011, 26, 2045–2051. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Wu, Y.; Hou, X.; Yuan, Z. Simultaneous determination of malachite green, gentian violet and their leuco-metabolites in aquatic products by high-performance liquid chromatography-linear ion trap mass spectrometry. J. Chromatogr. A 2007, 1172, 121–126. [Google Scholar] [PubMed]
- Ding, J.; Yang, S.; Liang, D.; Chen, H.; Wu, Z.; Zhang, L.; Ren, Y. Development of extractive electrospray ionization ion trap mass spectrometry for in vivo breath analysis. Analyst 2009, 134, 2040–2050. [Google Scholar]




| Sample | Amounts Added (mg·L−1) | Amounts Measured (mg·L−1) | Relative Standard Deviation (RSD, %) | Recovery (%) |
|---|---|---|---|---|
| Lake water | 0.100 | 0.115 | 6.64 | 115 |
| Fish water a | 0.0100 | 0.00854 | 9.17 | 85.4 |
| Lake water | 1.00 | 0.960 | 7.44 | 96.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Yang, S.; Chingin, K.; Zhu, L.; Zhang, X.; Zhou, Z.; Zhao, Z. Quantitative Detection of Trace Malachite Green in Aquiculture Water Samples by Extractive Electrospray Ionization Mass Spectrometry. Int. J. Environ. Res. Public Health 2016, 13, 814. https://doi.org/10.3390/ijerph13080814
Fang X, Yang S, Chingin K, Zhu L, Zhang X, Zhou Z, Zhao Z. Quantitative Detection of Trace Malachite Green in Aquiculture Water Samples by Extractive Electrospray Ionization Mass Spectrometry. International Journal of Environmental Research and Public Health. 2016; 13(8):814. https://doi.org/10.3390/ijerph13080814
Chicago/Turabian StyleFang, Xiaowei, Shuiping Yang, Konstantin Chingin, Liang Zhu, Xinglei Zhang, Zhiquan Zhou, and Zhanfeng Zhao. 2016. "Quantitative Detection of Trace Malachite Green in Aquiculture Water Samples by Extractive Electrospray Ionization Mass Spectrometry" International Journal of Environmental Research and Public Health 13, no. 8: 814. https://doi.org/10.3390/ijerph13080814
APA StyleFang, X., Yang, S., Chingin, K., Zhu, L., Zhang, X., Zhou, Z., & Zhao, Z. (2016). Quantitative Detection of Trace Malachite Green in Aquiculture Water Samples by Extractive Electrospray Ionization Mass Spectrometry. International Journal of Environmental Research and Public Health, 13(8), 814. https://doi.org/10.3390/ijerph13080814