Incidence and Simple Prediction Model of Hyperuricemia for Urban Han Chinese Adults: A Prospective Cohort Study
Abstract
:1. Background
2. Methods
2.1. Study Population
2.2. Biomarkers Selection and Measurements
2.3. Outcomes
2.4. Statistical Analysis
3. Results
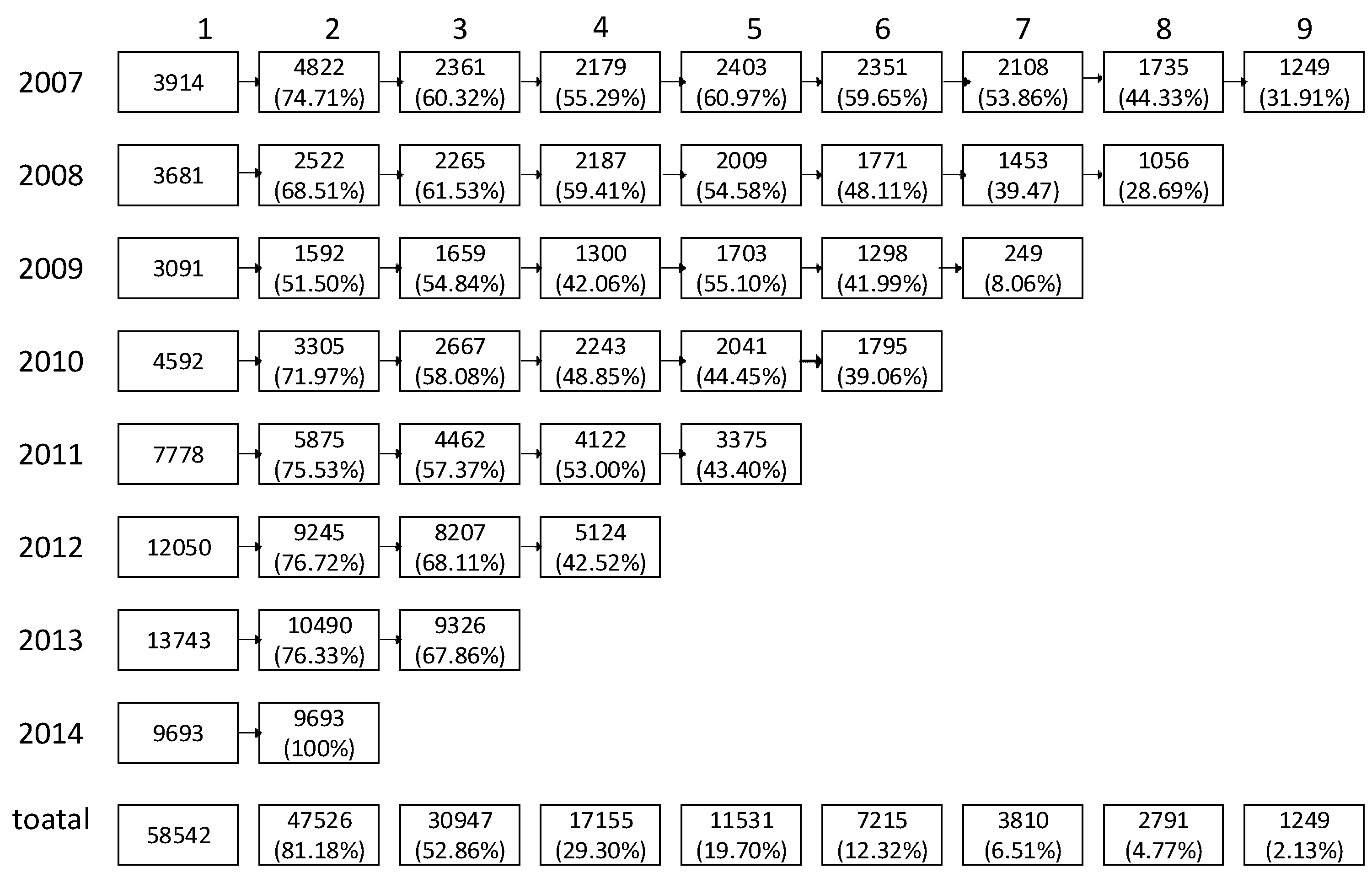
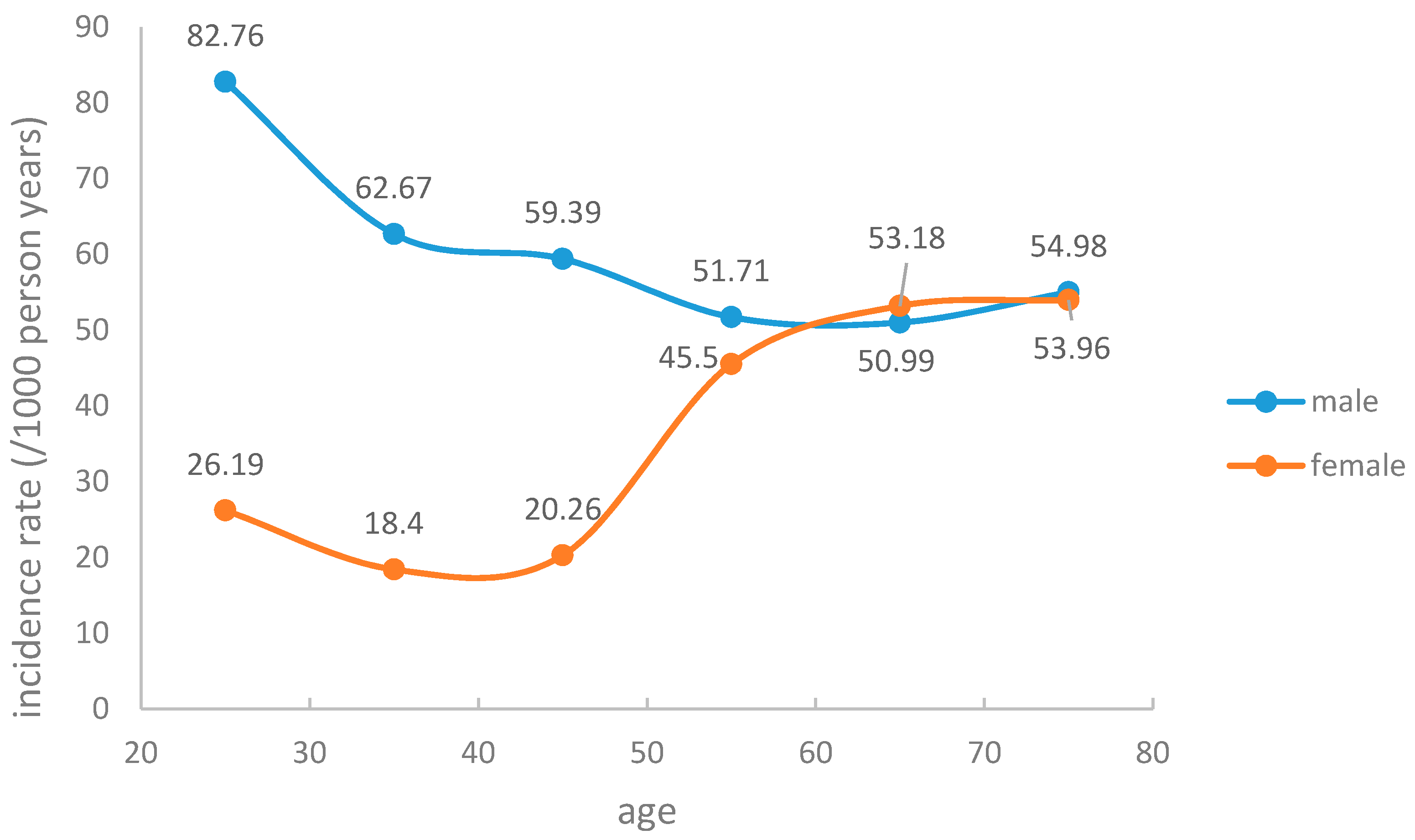
3.1. Hyperuricemia Incidence and Baseline Characteristics
3.2. Construction of Prediction Models
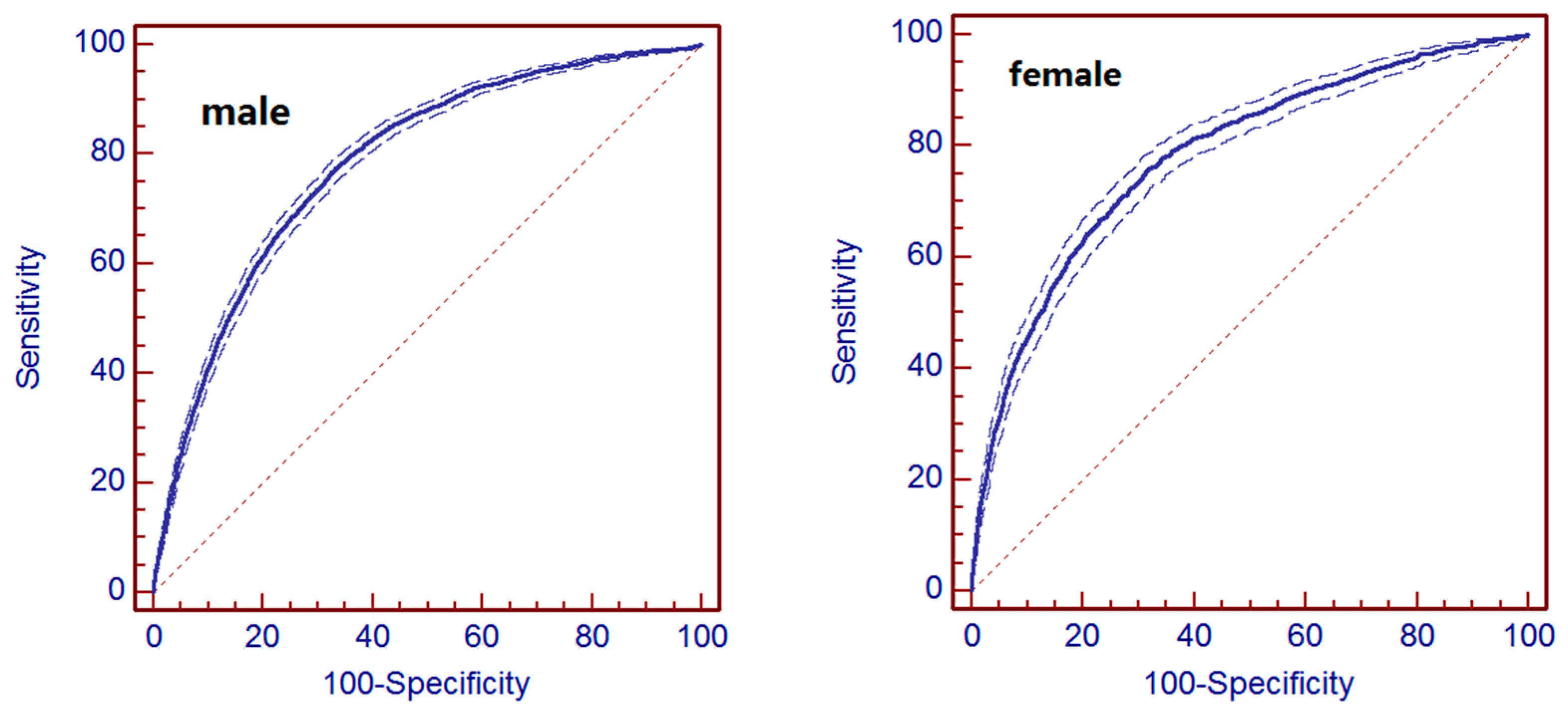
3.3. Evaluation of Prediction Models
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| SBP | systolic blood pressure |
| TG | triglycerides |
| CHOL | total cholesterol |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| SUA | serum uric acid |
| SCr | serum creatinine |
| ALT | alanine aminotransferase |
| HUA | Hyperuricemia |
| CI | Confidence interval |
References
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the U.S. general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Cheng, X.; Wu, J.; Liu, J.; Xu, T.; Ding, H.; Liu, Y.; Ge, Z.; Wang, Y.; Han, H. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 2013, 13, 356–410. [Google Scholar] [CrossRef] [PubMed]
- Lioté, F. Hyperuricemia and gout. Curr. Rheumatol. Rep. 2003, 5, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, C.; Zhao, Y.; Zeng, X.; Liu, F.; Fu, P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014, 15, 223–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, Y.; Chuang, Y.; Liao, S.; Lin, C.; Tang, Y.; Shu, W.H.; Chen, D. Relationship between hyperuricemia (HUC) and metabolic syndrome (MS) in institutionalized elderly men. Arch. Gerontol. Geriat. 2009, 49, 46–49. [Google Scholar] [CrossRef]
- Baker, J.F.; Krishnan, E.; Chen, L.; Schumacher, H.R. Serum uric acid and cardiovascular disease: Recent developments, and where do they leave us? Am. J. Med. 2005, 118, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lee, P.; Lei, C.; Hsu, Y.; Chang, H.; Tung, C.; Lin, C.; Yang, H.; Lu, L.; Jong, M. Hyperuricemia as an independent risk factor of chronic kidney disease in middle-aged and elderly population. Am. J. Med. Sci. 2010, 339, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.J.; Koudstal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M. Uric acid is a risk factor for myocardial infarction and stroke the Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Lin, H.; Chou, P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemia men in a prospective study. J. Rheumatol. 2000, 27, 1501–1505. [Google Scholar] [PubMed]
- McAdams-DeMarco, M.A.; Law, A.; Maynard, J.W.; Coresh, J.; Baer, A.N. Risk factors for incident hyperuricemia during mid-adulthood in African American and White men and women enrolled in the ARIC cohort study. BMC Musculoskelet. Disord. 2013, 14, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Tatara, K.; Nakamura, K.; Suzuki, K. Risk factors for the incidence of hyperuricaemia: A 6-year longitudinal study of middle-aged Japanese men. Int. J. Epidemiol. 1999, 28, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chang, Y.; Zhang, Y.; Kim, S.; Cho, J.; Son, H.J.; Shin, H.; Guallar, E. A cohort study of hyperuricemia in middle-aged South Korean men. Am. J. Epidemiol. 2012, 175, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, H.; Guo, X.; Zhang, X.; Zhou, Y.; Ou, Q.; Zheng, L.; Sun, Y. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: From lifestyle risk factors to metabolic comorbidities. Clin. Rheumatol. 2015, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Gao, Z.N.; Guo, X.H.; Hong, T.P.; Ji, Q.H.; Li, C.G.; Li, C.L.; Li, Q.; Li, M.; Li, Y.M.; et al. Guidelines for the diagnosis and treatment of hyperuricemia and gout. Chin. J. Endocrinol. Metab. 2013, 29, 913–920. [Google Scholar]
- Agostino, R.B.; Nam, B. Evaluation of the performance of survival analysis models: Discrimination and calibration measures. Handb. Stat. 2004, 23, 1–25. [Google Scholar]
- Norman, J.; Mueller, M.; Bien, M.; Vafaie, M.; Katus, H.A.; Giannitsis, E. Effect of older age on diagnostic and prognostic performance of high-sensitivity troponin T in patients presenting to an emergency department. Am. Heart J. 2012, 164, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Poletto, J.; Harima, H.A.; Ferreira, S.R.G.; Gimeno, S.G.A. Hyperuricemia and associated factors: A cross-sectional study of Japanese-Brazilians. Cad. Saúde Pública 2011, 27, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Tang, Z.; Fang, X.; Wu, X.; Liu, H.; Wang, C.; Hou, C. Prevalence of hyperuricemia among Beijing post-menopausal women in 10 years. Arch. Gerontol. Geriat. 2016, 64, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Pan, W.H.; Yeh, W.T.; Tsai, K.S. Hyperuricemia and gout in Taiwan: Results from the Nutritional and Health Survey in Taiwan (1993–1996). J. Rheumatol. 2001, 28, 1640–1645. [Google Scholar] [PubMed]
- Palmer, T.M.; Nordestgaard, B.G.; Benn, M.; Tybjærg-Hansen, A.; Smith, G.D.; Lawlor, D.A.; Timpson, N.J. Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. BMJ 2013, 347, 42–62. [Google Scholar] [CrossRef] [PubMed]
| Variables | Males | p-Value | Females | p-Value | ||
|---|---|---|---|---|---|---|
| HUA | Non-HUA | HUA | Non-HUA | |||
| (n = 5581) | (n = 29,395) | (n = 1554) | (n = 22,008) | |||
| Age (years) | 41.69 ± 12.91 | 43.85 ± 13.42 | <0.0001 | 45.48 ± 14.59 | 41.21 ± 12.41 | <0.0001 |
| SBP (mmHg) | 132.6 ± 17.37 | 130.4 ± 17.32 | <0.0001 | 127.7 ± 21.18 | 120.5 ± 18.11 | <0.0001 |
| BMI (kg/m2) | 25.98 ± 3.12 | 24.91 ± 3.23 | <0.0001 | 24.35 ± 3.57 | 22.61 ± 3.21 | <0.0001 |
| SUA (µmol/L) | 369.4 ± 40.94 | 320.5 ± 52.86 | <0.0001 | 294.7 ± 45.21 | 245.0 ± 47.89 | <0.0001 |
| CHOL (mmol/L) | 4.97 ± 0.93 | 4.83 ± 0.90 | <0.0001 | 5.06 ± 1.02 | 4.75 ± 0.93 | <0.0001 |
| TG (mmol/L) | 1.91 ± 1.56 | 1.54 ± 1.26 | <0.0001 | 1.41 ± 0.92 | 1.07 ± 0.77 | <0.0001 |
| HDL-C (mmol/L) | 1.31 ± 0.31 | 1.35 ± 0.32 | <0.0001 | 1.47 ± 0.33 | 1.54 ± 0.35 | <0.0001 |
| LDL-C (mmol/L) | 2.95 ± 0.73 | 2.86 ± 0.72 | <0.0001 | 2.95 ± 0.81 | 2.68 ± 0.75 | <0.0001 |
| SCr (µmol/L) | 71.25 ± 13.63 | 70.04 ± 14.91 | <0.0001 | 54.35 ± 10.61 | 52.97 ± 11.18 | <0.0001 |
| ALT (U/L) | 30.31 ± 23.31 | 26.62 ± 21.95 | <0.0001 | 20.47 ± 13.89 | 17.30 ± 14.86 | <0.0001 |
| Variables | Sample Characteristics | HR (95% CI) | p-Value |
|---|---|---|---|
| Males | |||
| Age (years) | 43.50 (13.36) | 0.990 (0.988–0.992) | <0.0001 |
| SBP (mmHg) | 130.75 (17.34) | 1.009 (1.008–1.011) | <0.0001 |
| BMI (kg/m2) | 25.08 (3.23) | 1.101 (1.093–1.109) | <0.0001 |
| SUA (µmol/L) | 328.35 (54.18) | 1.022 (1.022–1.023) | <0.0001 |
| CHOL (mmol/L) | 4.86 (0.91) | 1.156 (1.124–1.189) | <0.0001 |
| TG (mmol/L) | 1.60 (1.32) | 1.105 (1.093–1.118) | <0.0001 |
| HDL-C (mmol/L) | 1.35 (0.32) | 0.741 (0.681–0.806) | 0.0004 |
| LDL-C (mmol/L) | 2.87 (0.72) | 1.217 (1.171–1.261) | <0.0001 |
| SCr (µmol/L) | 70.23 (14.72) | 1.006 (1.005–1.006) | <0.0001 |
| ALT (U/L) | 27.21 (22.21) | 1.004 (1.003–1.004) | <0.0001 |
| Females | |||
| Age (years) | 41.49 (12.61) | 1.025(1.021–1.029) | <0.0001 |
| SBP (mmHg) | 120.93 (18.42) | 1.014 (1.011–1.017) | <0.0001 |
| BMI (kg/m2) | 22.72 (3.26) | 1.095 (1.085–1.106) | <0.0001 |
| SUA (µmol/L) | 248.28 (49.28) | 1.023 (1.022–1.024) | <0.0001 |
| CHOL (mmol/L) | 4.77 (0.94) | 1.160 (1.098–1.224) | 0.0034 |
| TG (mmol/L) | 1.08 (0.78) | 1.161 (1.130–1.193) | <0.0001 |
| HDL-C (mmol/L) | 1.53 (0.35) | 0.653 (0.565–0.754) | 0.0019 |
| LDL-C (mmol/L) | 2.70 (0.76) | 1.320 (1.237–1.410) | <0.0001 |
| SCr (µmol/L) | 53.06 (11.15) | 1.005 (1.003–1.006) | 0.0209 |
| ALT (U/L) | 17.51 (14.82) | 1.004 (1.003–1.006) | <0.0001 |
| Variables | β Coefficient | SE | HR (95% CI) | p-Value |
|---|---|---|---|---|
| Males | ||||
| Age (years) | −0.00891 | 0.0012 | 0.991 (0.989–0.993) | <0.0001 |
| SBP (mmHg) | 0.00445 | 0.0010 | 1.004 (1.003–1.006) | <0.0001 |
| BMI (kg/m2) | 0.03744 | 0.0054 | 1.042 (1.033–1.051) | <0.0001 |
| SUA (µmol/L) | 0.02095 | 0.0004 | 1.022 (1.021–1.022) | <0.0001 |
| Females | ||||
| BMI (kg/m2) | 0.04934 | 0.0071 | 1.051 (1.036–1.065) | <0.0001 |
| SBP (mmHg) | 0.00523 | 0.0016 | 1.005 (1.003–1.008) | <0.0001 |
| SUA (µmol/L) | 0.02196 | 0.0006 | 1.022 (1.021–1.023) | <0.0001 |
| TG (mmol/L) | 0.04633 | 0.0235 | 1.047 (1.000–1.097) | 0.0483 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wang, C.; Zhang, G.; Ji, X.; Liu, Y.; Sun, X.; Yuan, Z.; Jiang, Z.; Xue, F. Incidence and Simple Prediction Model of Hyperuricemia for Urban Han Chinese Adults: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 67. https://doi.org/10.3390/ijerph14010067
Cao J, Wang C, Zhang G, Ji X, Liu Y, Sun X, Yuan Z, Jiang Z, Xue F. Incidence and Simple Prediction Model of Hyperuricemia for Urban Han Chinese Adults: A Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2017; 14(1):67. https://doi.org/10.3390/ijerph14010067
Chicago/Turabian StyleCao, Jin, Chunxia Wang, Guang Zhang, Xiang Ji, Yanxun Liu, Xiubin Sun, Zhongshang Yuan, Zheng Jiang, and Fuzhong Xue. 2017. "Incidence and Simple Prediction Model of Hyperuricemia for Urban Han Chinese Adults: A Prospective Cohort Study" International Journal of Environmental Research and Public Health 14, no. 1: 67. https://doi.org/10.3390/ijerph14010067





