Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biochar Preparation
2.3. Biochar Characterization
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of Biochar
3.1.1. SEM and BET Analysis
3.1.2. Elemental Analysis
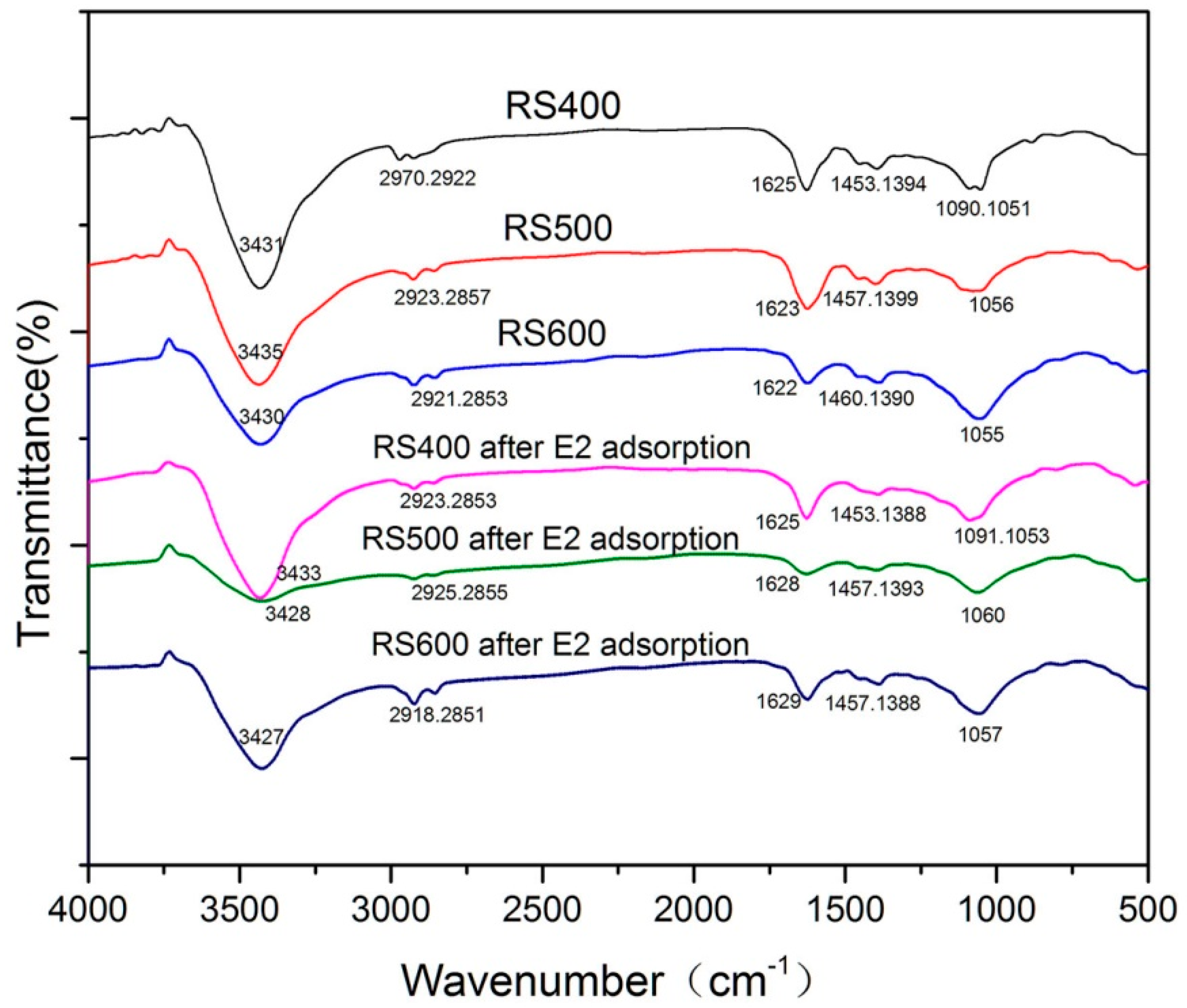
3.1.3. FTIR Analysis
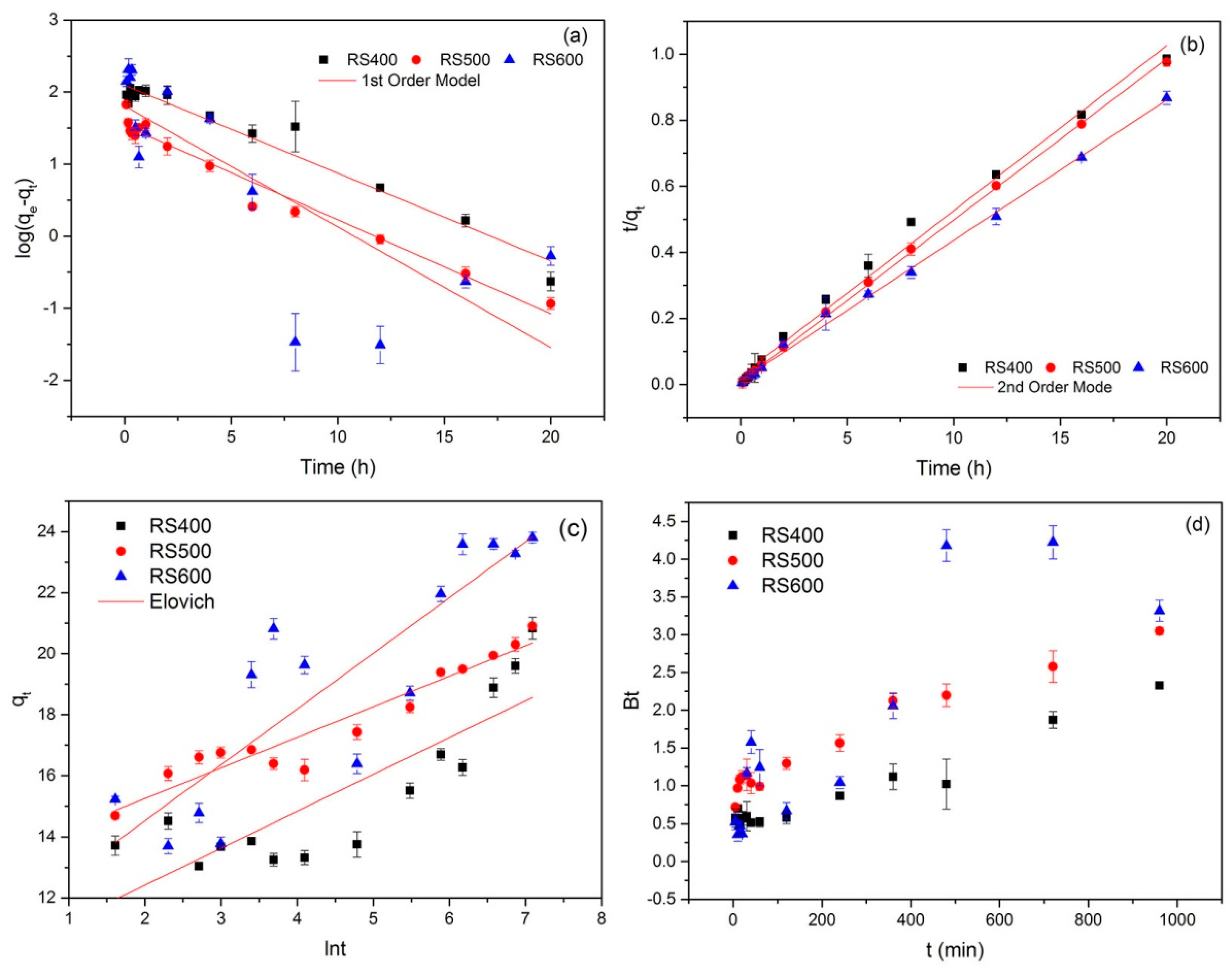
3.2. Adsorption Kinetics
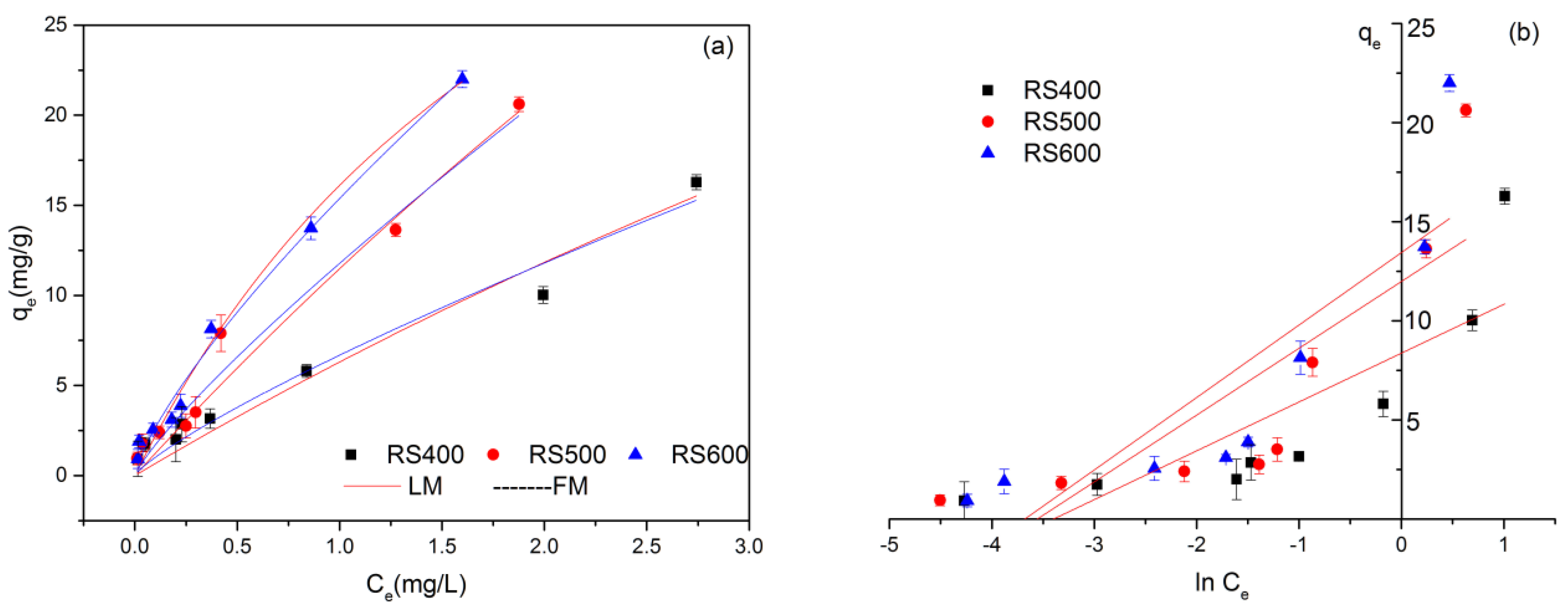
3.3. Adsorption Isotherms
3.4. Effect of Solution pH
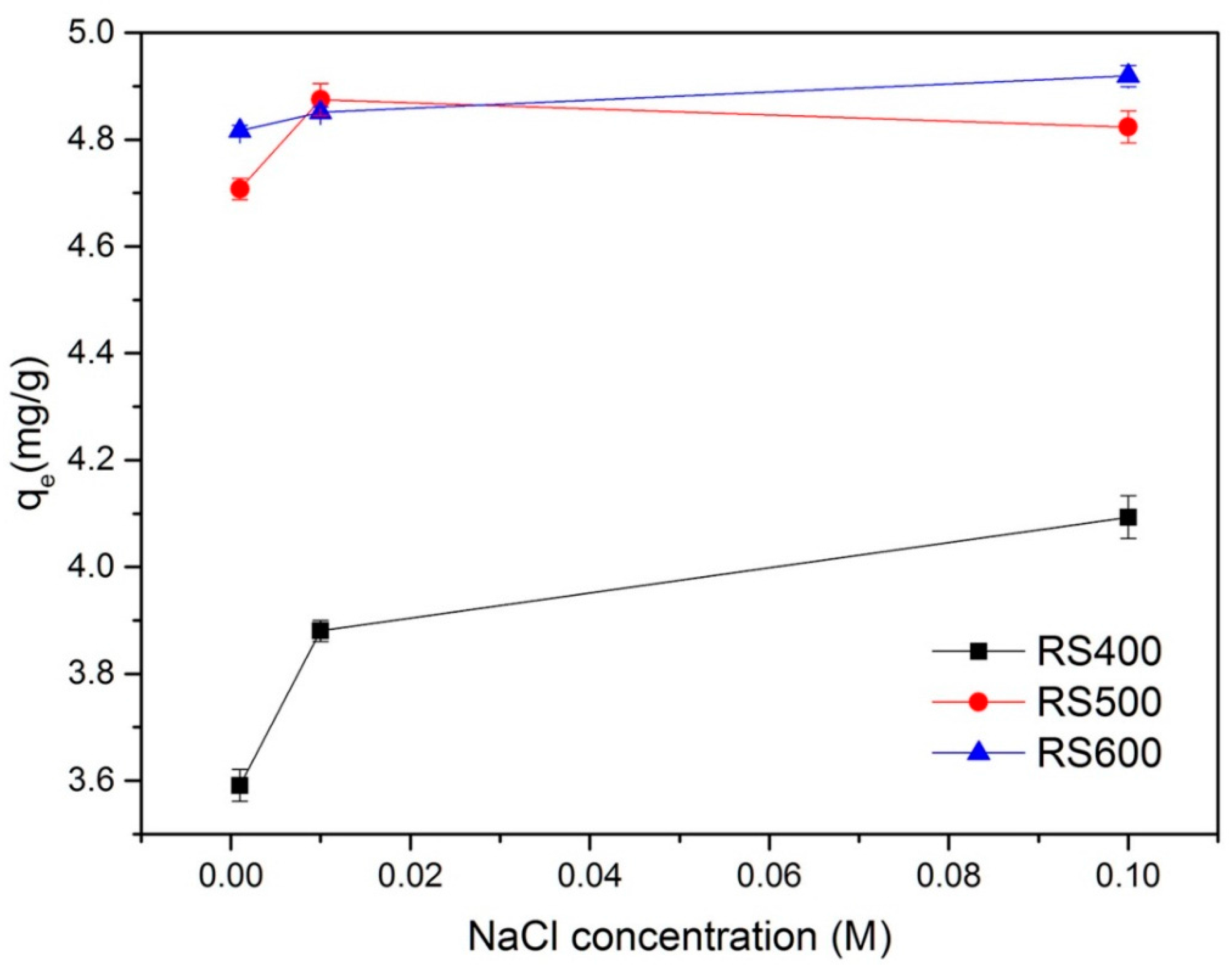
3.5. Effect of Ionic Strength
3.6. Effect of Background Electrolyte
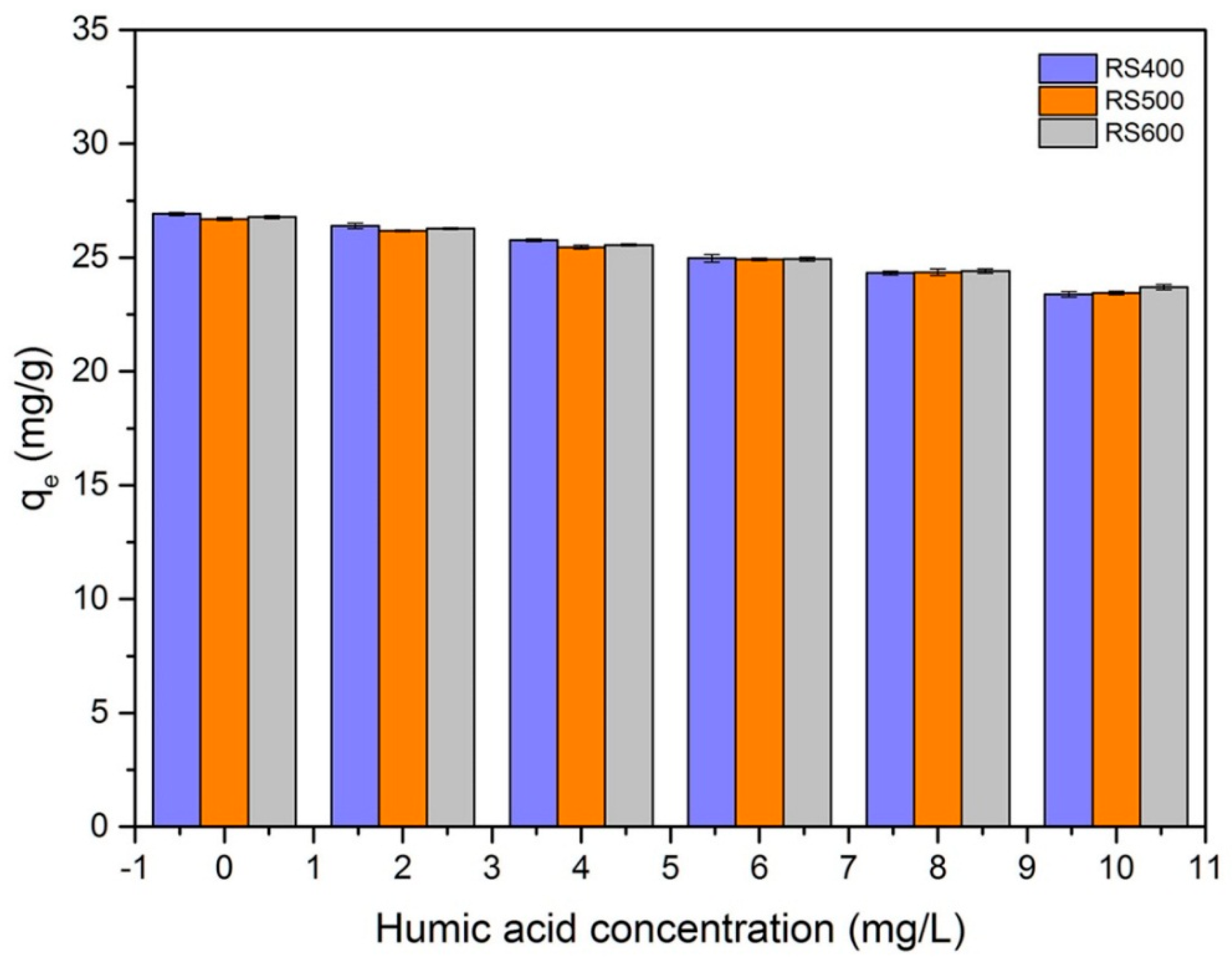
3.7. Effect of Humic Acid
3.8. Possible Mechanisms
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shore, L.S.; Gurevitz, M.; Shemesh, M. Estrogen as an environmental pollutant. Bull. Environ. Contam. Toxicol. 1993, 51, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Crisp, T.M.; Clegg, E.D.; Cooper, R.L.; Wood, W.P.; Anderson, D.G.; Baetcke, K.P.; Hoffmann, J.L.; Morrow, M.S.; Rodier, D.J.; Schaeffer, J.E. Special Report on Environmental Endocrine Disruption: An Effect Assessment and Analysis. Environ. Health Perspect. 1998, 106 (Suppl. 1), 11–56. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Dumont, E.; Williams, R.J.; Oldenkamp, R.; Cisowska, I.; Sumpter, J.P. Do concentrations of ethinylestradiol, estradiol, and diclofenac in European rivers exceed proposed EU environmental quality standards? Environ. Sci. Technol. 2013, 47, 12297–12304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.D.; Bartkow, M.; Blockwell, S.J.; Coleman, H.M.; Khan, S.J.; Lim, R.; Mcdonald, J.A.; Nice, H.; Nugegoda, D.; Pettigrove, V. An assessment of endocrine activity in Australian rivers using chemical and in vitro analyses. Environ. Sci. Pollut. Res. Int. 2014, 21, 12951–12967. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Domingues, V.F.; Pinho, C.; Fernandes, V.C.; Delerue-Matos, C.; Gameiro, P.; Mansilha, C. Occurrence of Bisphenol A, Estrone, 17β-Estradiol and 17α-Ethinylestradiol in Portuguese Rivers. Bull. Environ. Contam. Toxicol. 2013, 90, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Hassani, G.; Babaei, A.; Takdastan, A.; Shirmardi, M.; Yousefian, F. Occurrence and fate of 17β-estradiol in water resources and wastewater in Ahvaz, Iran. Glob. Nest 2016, 18, 855–866. [Google Scholar]
- Kim, S.-H.; Tian, Q.; Fang, J.; Sung, S. Removal of 17-β estradiol in water by sonolysis. Int. Biodeterior. Biodegrad. 2015, 102, 11–14. [Google Scholar] [CrossRef]
- Tanaka, H.; Yakou, Y.; Takahashi, A.; Higashitani, T.; Komori, K. Comparison between estrogenicities estimated from DNA recombinant yeast assay and from chemical analyses of endocrine disruptors during sewage treatment. Water Sci. Technol. 2001, 43, 125–132. [Google Scholar] [PubMed]
- Chang, H.S.; Choo, K.H.; Lee, B.; Choi, S.J. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—Physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Zaib, Q.; Khan, I.A.; Saleh, N.B.; Flora, J.R.V.; Park, Y.-G.; Yoon, Y. Removal of Bisphenol A and 17β-Estradiol by Single-Walled Carbon Nanotubes in Aqueous Solution: Adsorption and Molecular Modeling. Water Air Soil Pollut. 2012, 223, 3281–3293. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, K. Adsorption of 17β-estradiol by multi-walled carbon nanotubes in natural waters with or without aquatic colloids. Chem. Eng. J. 2014, 258, 185–193. [Google Scholar] [CrossRef]
- Li, Z.; Dvorak, B.; Li, X. Removing 17β-estradiol from drinking water in a biologically active carbon (BAC) reactor modified from a granular activated carbon (GAC) reactor. Water Res. 2012, 46, 2828–2836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.L. Removal of estrone and 17β-estradiol from water by adsorption. Water Res. 2005, 39, 3991–4003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Zeng, G.; Xiao, F.; Hu, X.; Hu, X.; Wang, H.; Li, T.; Zhou, L.; Tan, X. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, S.; Zeng, G.; Hu, X.; Hu, X.; Guo, Z.; Tan, X.; Wang, L.; Wu, Z. Adsorption of Estrogen Contaminants by Graphene Nanomaterials under Natural Organic Matter Preloading: Comparison to Carbon Nanotube, Biochar, and Activated Carbon. Environ. Sci. Technol. 2017, 51, 6352–6359. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gua, Y.; Yanga, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Sewu, D.D.; Boakye, P.; Woo, S.H. Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Bioresour. Technol. 2017, 224, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.M.; Amer, M.E.; Elmarsafy, A.E.; Elkady, M.Y. Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water. J. Environ. Chem. Eng. 2014, 2, 2013–2025. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(ii) and Pb(ii) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hua, X.; Jiang, L.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Sangeeta, P.; Han, J.; Gao, W. Sorption of 17β-estradiol from aqueous solutions on to bone char derived from waste cattle bones: Kinetics and isotherms. J. Environ. Chem. Eng. 2015, 3, 1562–1569. [Google Scholar]
- Li, P.C.; Wei, W.; Zhang, F.S.; Wang, D.Y.; Jie, H. Structural characteristics of straw biochars and sorption of 17β-estradiol on straw biochar. Res. Environ. Sci. 2015. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, S.; Hu, X.; Zeng, G.; Hu, X.; Liu, S.; Liu, S.; Huang, B.; Li, M. Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem. Eng. J. 2016, 308, 597–605. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.Y.; Cho, T.S.; Choi, J.W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Yi, W.; Bai, X.; Li, Z.; Hu, S.; Xiang, J. Effect of temperature on gas composition and char structural features of pyrolyzed agricultural residues. Bioresour. Technol. 2011, 102, 8211–8219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kazi, Z.H.; Schnitzer, M.I.; Monreal, C.M.; Mayer, P. Separation and identification of heterocyclic nitrogen compounds in biooil derived by fast pyrolysis of chicken manure. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2011, 46, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Michael Jerry Antal, J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Sheth, A.C.; Bagchi, B. Investigation of Nitrogen-Bearing Species in Catalytic Steam Gasification of Poultry Litter. J. Air Waste Manag. Assoc. 2005, 55, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.S.; Cantrell, K.B.; Hunt, P.G. High-Temperature Pyrolysis of Blended Animal Manures for Producing Renewable Energy and Value-Added Biochar. Ind. Eng. Chem. Res. 2010, 49, 10125–10131. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lua, H.; Chena, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Joshi, S.; Karna, A.K. Analysis of Phytoconstiuents and Cytotoxic Activities of Different Parts of Ocimum sanctum. Int. J. Appl. Sci. Biotechnol. 2013, 1, 137–144. [Google Scholar] [CrossRef]
- Kavitha, R.; Rajarajeswari, G.R. Preparation, characterization and bioactivity assessment of chitosan-acetylated jute blended green films. Fibers Polym. 2013, 14, 1454–1459. [Google Scholar] [CrossRef]
- Shakshooki, S.K.; EL-Tarhuni, S.R.; EL-Hamady, A.M. Synthesis and Characterization of Pellicular γ-Zirconium Phosphate Fibrous Cerium Phosphate Nanocomposite Membranes. Am. J. Chem. 2014, 4, 109–115. [Google Scholar]
- Liang, L.; Rieke, P.C.; Liu, J.; Fryxell, G.E.; Young, J.S.; Engelhard, M.H.; Alford, K.L. Surfaces with Reversible Hydrophilic/Hydrophobic Characteristics on Cross-linked Poly(N-isopropylacrylamide) Hydrogels. Langmuir 2000, 16, 8016–8023. [Google Scholar] [CrossRef]
- Gomaa, M.; Fattah, G.A. Synthesis of Graphene and Graphene Oxide by Microwave Plasma Chemical Vapor Deposition. J. Am. Sci. 2016, 12, 72–80. [Google Scholar]
- Charles, J.; Muthusamy, S. Comparative Study of Butyl Rubber (IIR) and Bromobutyl Rubber (BIIR) based on FTIR, Dielectric and Thermal Studies. J. Appl. Sci. Eng. Methodol. 2016, 2, 206–211. [Google Scholar]
- Wang, J.; Li, Q.; Li, M.M.; Chen, T.H.; Zhou, Y.F.; Yue, Z.B. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria. Bioresour. Technol. 2014, 163, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Xuemei, H.; Hao, Y. Fabrication of Polystyrene/Detonation Nanographite Composite Microspheres with the Core/Shell Structure via Pickering Emulsion Polymerization. J. Nanomater. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhao, S.C.; Liu, Z.Y.; Rui, R.S.; Qiu, W.B.; Guo, Y.Q.; Li, M.J.; Yang, W.T.; Cai, C.B. Rapid thermal decomposition for YBa2Cu3O7−δfilms derived by DEA-modified TFA-MOD. J. Phys. Conf. Ser. 2014, 507. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. A green approach for the reduction of graphene oxide by wild carrot root. Carbon 2012, 50, 914–921. [Google Scholar] [CrossRef]
- Yimer, A.M. Review on Preparation and Description of Some First Series Divalent Transition Metal Complexes with Novel Schiff’s Base Ligands. Rev. Catal. 2015, 2, 14–25. [Google Scholar] [CrossRef]
- Nityanandi, D.; Subbhuraam, C.V. Kinetics and thermodynamic of adsorption of chromium(VI) from aqueous solution using puresorbe. J. Hazard. Mater. 2009, 170, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Özacar, M.; Şengil, İ.A. A kinetic study of metal complex dye sorption onto pine sawdust. Process Biochem. 2005, 40, 565–572. [Google Scholar] [CrossRef]
- Boyd, G.E.; Myers, L.S., Jr.; Adamson, A.W. The exchange adsorption of ions from aqueous solutions by organic zeolites; performance of deep adsorbent beds under non-equilibrium conditions. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Djeribi, R.; Hamdaoui, O. Sorption of copper(II) from aqueous solutions by cedar sawdust and crushed brick. Desalination 2008, 225, 95–112. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C.; Al-Muhtaseb, A.A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 217–222. [Google Scholar]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.; Karanfil, T. Adsorption of synthetic organic chemicals by carbon nanotubes: Effects of background solution chemistry. Water Res. 2010, 44, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Liu, Y.G.; Zeng, G.M.; Liu, S.B.; Shu, D.; Hu, X.J.; Jiang, L.H.; Tan, X.F.; Cai, X.X.; Yan, Z.L. Tetracycline absorbed onto nitrilotriacetic acid-functionalized magnetic graphene oxide: Influencing factors and uptake mechanism. J. Colloid Interface Sci. 2017, 485, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Amy, A.; Childress, E.; Elimelech, M. Relating Nanofiltration Membrane Performance to Membrane Charge (Electrokinetic) Characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar]
- Uğurlu, M. Adsorption of a textile dye onto activated sepiolite. Microporous Mesoporous Mater. 2009, 119, 276–283. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef] [PubMed]


| Samples | C (%) | N (%) | S (%) | H (%) | C/N |
|---|---|---|---|---|---|
| RS400 | 51.62 | 0.720 | 0.224 | 2.821 | 71.67 |
| RS500 | 58.75 | 0.777 | 0.246 | 2.176 | 75.6 |
| RS600 | 56.11 | 0.416 | 0.219 | 1.804 | 135 |
| Models | RS400 | RS500 | RS600 | |
|---|---|---|---|---|
| Pseudo-first-order | qe (mg g−1) | 8.108 | 4.639 | 6.090 |
| k1 (1 h−1) | 0.121 | 0.130 | 0.167 | |
| R2 | 0.955 | 0.967 | 0.605 | |
| Pseudo-second-order | qe (mg g−1) | 20.269 | 20.693 | 23.942 |
| k2 (g mg−1 h−1) | 0.088 | 0.215 | 0.131 | |
| R2 | 0.990 | 0.998 | 0.997 | |
| Elovich | α | 4853.07 | 553,319.39 | 703.32 |
| β | 0.829 | 0.998 | 0.547 | |
| R2 | 0.691 | 0.914 | 0.736 | |
| Models | RS400 | RS500 | RS600 | |
|---|---|---|---|---|
| Freundlich | Kf (L mg−1) | 6.858 | 12.044 | 13.952 |
| n | 0.779 | 0.812 | 0.764 | |
| R2 | 0.961 | 0.969 | 0.947 | |
| Langmuir | qm (mg g−1) | 59.890 | 64.888 | 52.994 |
| KL (L mg−1) | 0.124 | 0.237 | 0.374 | |
| R2 | 0.945 | 0.965 | 0.935 | |
| Temkin | A | 29.877 | 35.005 | 39.534 |
| B | 2.463 | 3.370 | 3.655 | |
| R2 | 0.638 | 0.632 | 0.682 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, N.; Liu, Y.; Jiang, L.; Zeng, G.; Tan, X.; Liu, S.; Yin, Z.; Tian, S.; Li, J. Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry. Int. J. Environ. Res. Public Health 2017, 14, 1213. https://doi.org/10.3390/ijerph14101213
Wang X, Liu N, Liu Y, Jiang L, Zeng G, Tan X, Liu S, Yin Z, Tian S, Li J. Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry. International Journal of Environmental Research and Public Health. 2017; 14(10):1213. https://doi.org/10.3390/ijerph14101213
Chicago/Turabian StyleWang, Xiaohua, Ni Liu, Yunguo Liu, Luhua Jiang, Guangming Zeng, Xiaofei Tan, Shaobo Liu, Zhihong Yin, Sirong Tian, and Jiang Li. 2017. "Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry" International Journal of Environmental Research and Public Health 14, no. 10: 1213. https://doi.org/10.3390/ijerph14101213





