Presence of Legionella spp. in Hot Water Networks of Different Italian Residential Buildings: A Three-Year Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Inspections
2.2. Sampling
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
3.1. Inspections and Physical-Chemical Results
3.2. Drinking Water Parameters Results
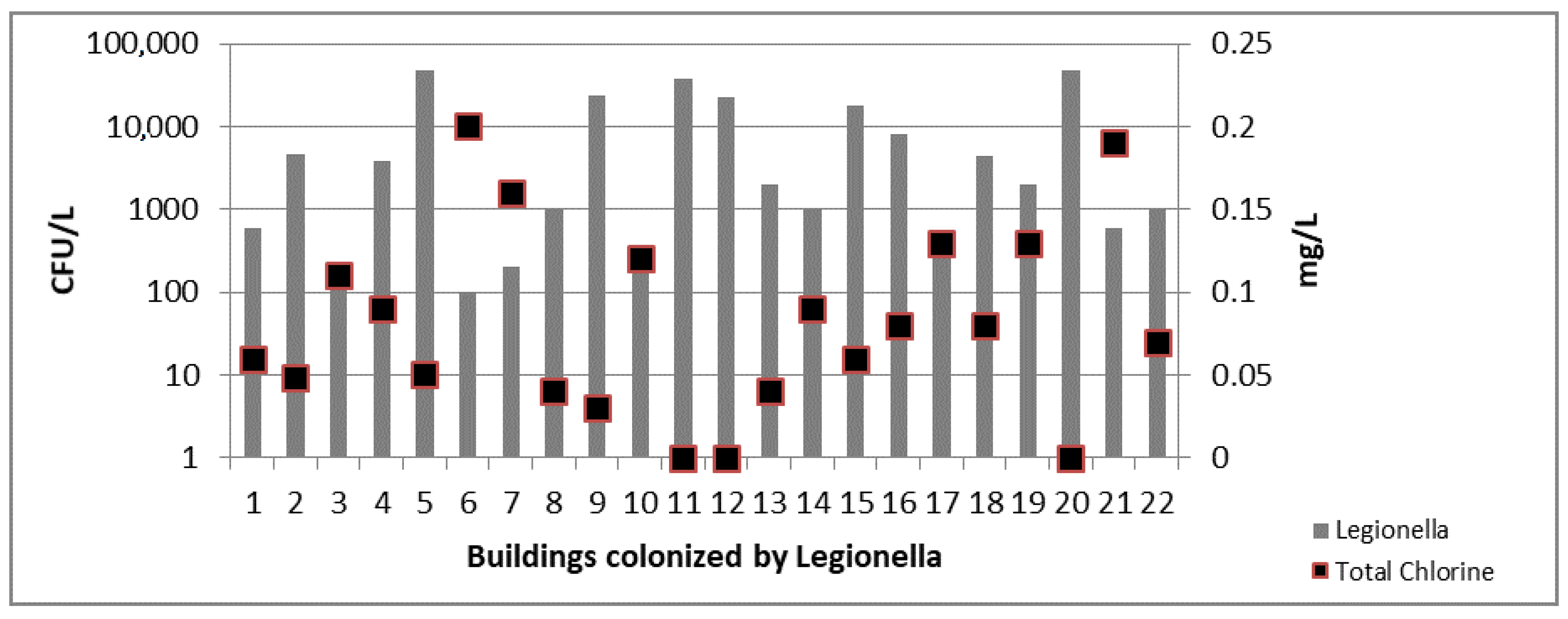
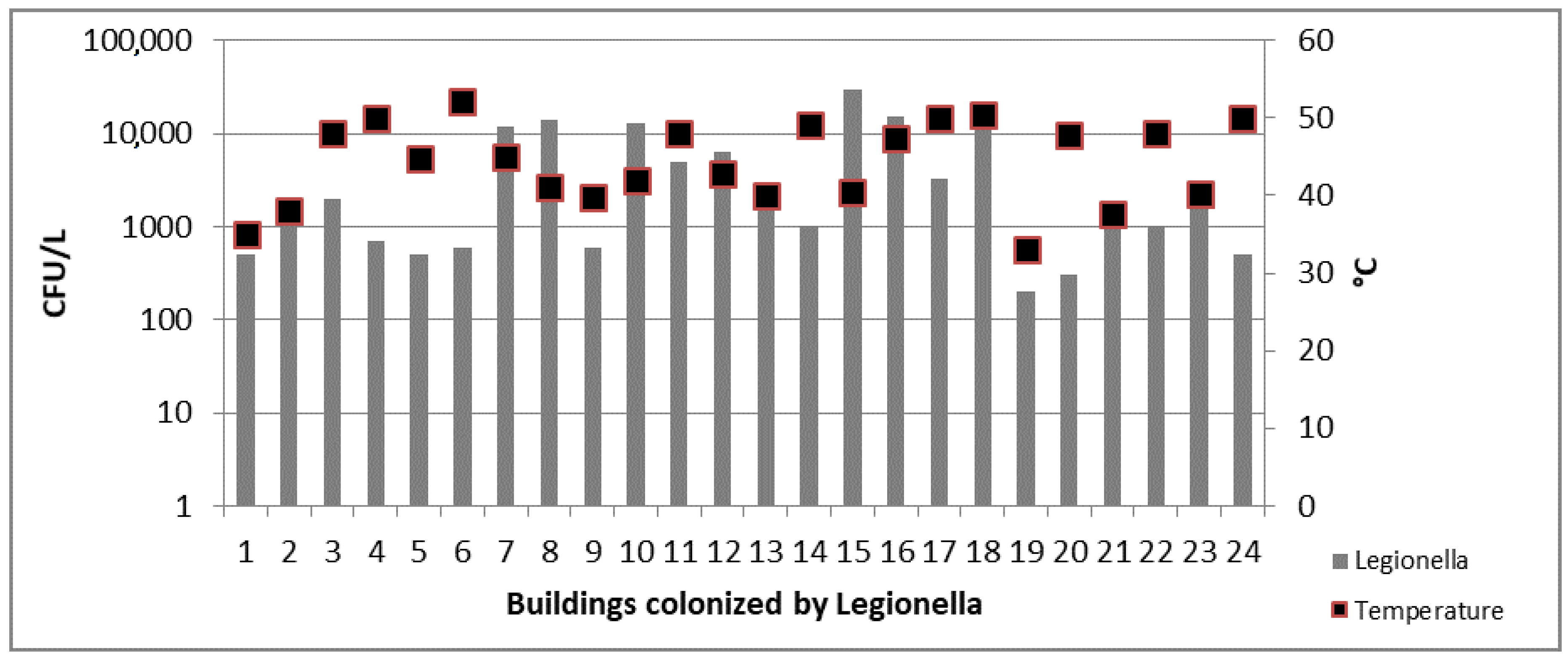
3.3. Legionella spp. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C.W. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 1994, 60, 1585–1592. [Google Scholar] [PubMed]
- Bates, M.N.; Maas, E.; Martin, T.; Harte, D.; Grubner, M.; Margolin, T. Investigation of the prevalence of Legionella species in domestic hot water systems. N. Z. Med. J. 2000, 113, 218–220. [Google Scholar] [PubMed]
- Fitzgeorge, R.B.; Baskerville, A.; Broster, M.; Hambleton, P.; Dennis, P.J. Aerosol infection of animals with strains of Legionella pneumophila of different virulence: Comparison with intraperitoneal and intranasal routes of infection. Epidemiol. Infect. 1983, 90, 81–89. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaire’s disease: 25 years of investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, M.H.; Yu, V.L.; Muder, R.R. Legionnaires’ disease in long-term care facilities: Overview and proposed solutions. J. Am. Geriatr. Soc. 2005, 53, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Diederen, B.M.W. Legionella spp. and Legionnaires’ disease. J. Infect. 2008, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Italian National Institute of Health. Rapporto Annuale Sulla Legionellosi in Italia Nel 2015; Italian National Institute of Health: Rome, Italy, 2016; Volume 29, pp. 1–28. [Google Scholar]
- European Centre for Disease Prevention and Control. Legionnaires’ Disease–Annual Epidemiological Report for 2015; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2017; pp. 1–36. [Google Scholar]
- Casini, B.; Buzzigoli, A.; Cristina, M.L.; Spagnolo, A.M.; Del Giudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Valentini, P.; Baggiani, A.; et al. Long-term effects of hospital water network disinfection on Legionella and other waterborne bacteria in an Italian university hospital. Infect. Control Hosp. Epidemiol. 2014, 35, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Spagnolo, A.M.; Casini, B.; Baggiani, A.; Del Giudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Perdelli, F.; Orlando, P. The impact of aerators on water contamination by emerging gram-negative opportunists in at-risk hospital departments. Infect. Control Hosp. Epidemiol. 2014, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H. Legionella risk management and control in potable water systems: Argument for the abolishment of routine testing. Int. J. Environ. Res. Public Health 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Fasano, F.; Iatta, R.; Barbuti, G.; Cuna, T.; Montagna, M.T. Legionella spp. and legionellosis in southeastern Italy: Disease epidemiology and environmental surveillance in community and health care facilities. BMC Public Health 2010, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Mathys, W.; Stanke, J.; Harmuth, M.; Junge-Mathys, E. Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int. J. Hyg. Environ. Health 2008, 211, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Uzel, A.; Uçar, F.; Hameş-Kocabaş, E.E. Prevalence of Legionella pneumophila serogroup 1 in water distribution systems in Izmir province of Turkey. APMIS 2005, 113, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Pryor, M.; Fields, B.; Lucas, C.; Phelan, M.; Besser, R.E. Introduction of monochloramine into a municipal water system: Impact on colonization of buildings by Legionella spp. Appl. Environ. Microbiol. 2006, 72, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Pringler, N.; Brydov, P.; Uldum, S. Occurrence of Legionella in Danish hot water systems. In Legionella; Marre, R., Abu Kwaik, Y., Bartlett, C., Cianciotto, N., Fields, B., Frosch, M., Hacker, J., Lück, P., Eds.; ASM: Washington, DC, USA, 2002; pp. 298–301. [Google Scholar]
- Stojek, N.M.; Dutkiewicz, J. Co-existence of Legionella and other Gram-negative bacteria in potable water from various rural and urban sources. Ann. Agric. Environ. Med. 2011, 18, 330–334. [Google Scholar] [PubMed]
- Italian National Institute of Health. Linee Guida per la Prevenzione ed il Controllo della Legionellosi; Italian National Institute of Health: Rome, Italy, 2015. [Google Scholar]
- The Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption; The Council of the European Union: Bruxelles, Belgium, 1998; pp. 1–23. [Google Scholar]
- Baggiani, A.; Casini, B.; Totaro, M.; Aquino, F.; Valentini, P.; Bruni, B.; Porretta, A.; Casalini, F.; Miccoli, M.; Privitera, G. Colonization by Legionella spp. of water networks in residential buildings of the province of Pisa, Italy. Ann. Ig. 2014, 27, 718–725. [Google Scholar]
- International Organization for Standardization. ISO 6222 Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium; International Organization for Standardization: Geneva, Switzerland, 2001; pp. 1–4. [Google Scholar]
- International Organization for Standardization. ISO 9308-2 Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 2: Most Probable Number Method; International Organization for Standardization: Geneva, Switzerland, 2012; pp. 1–45. [Google Scholar]
- International Organization for Standardization. ISO 7899-2 Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method; International Organization for Standardization: Geneva, Switzerland, 2000; pp. 1–7. [Google Scholar]
- International Organization for Standardization. ISO 11731 Water Quality—Detection and Enumeration of Legionella; International Organization for Standardization: Geneva, Switzerland, 1998; pp. 1–38. [Google Scholar]
- Garrison, L.E.; Kunz, J.M.; Cooley, L.A.; Moore, M.R.; Lucas, C.; Schrag, S.; Sarisky, J.; Whitney, C.G. Vital signs: Deficiencies in environmental control identified in outbreaks of Legionnaires’ disease-North America, 2000–2014. Am. J. Transplant. 2016, 16, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Borella, P.; Montagna, M.T.; Romano-Spica, V.; Stampi, S.; Stancanelli, G.; Triassi, M.; Neglia, R.; Marchesi, I.; Fantuzzi, G.; Tatò, D.; et al. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 2004, 10, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; De Luca, G.; Legnani, P.P.; Sacchetti, R.; Stampi, S.; Zanetti, F. Legionella waterline colonization: Detection of Legionella species in domestic, hotel and hospital hot water systems. J. Appl. Microbiol. 2005, 98, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Yang, K.; Chun, B.C. Community-acquired Legionnaires’ disease in a newly constructed apartment building. J. Prev. Med. Public Health 2017, 50, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadi, D.; Velonakis, E. Detection of Legionella spp. from domestic water in the prefecture of Arta, Greece. J. Pathog. 2014, 2014, 407385. [Google Scholar] [PubMed]
- Zietz, B.; Wiese, J.; Brengelmann, F.; Dunkelberg, H. Presence of Legionellaceae in warm water supplies and typing of strains by polymerase chain reaction. Epidemiol. Infect. 2001, 126, 147–152. [Google Scholar] [PubMed]


| Physical-Chemical Parameters | Hot Water Samples | Cold Water Samples | |||
|---|---|---|---|---|---|
| Point A | Point B | Point I | Point E | Point T | |
| Building with independent hot water production (IB) | |||||
| Total Chlorine (mg/L) | 0.21 ± 1.1 | 0.15 ± 0.07 | 0.05 ± 0.03 | 0.17 ± 0.08 | 0.11 ± 0.05 |
| pH | 6.4 ± 0.8 | 6.6 ± 1.2 | NA | NA | NA |
| Conductivity (µS) | 801 ± 253 | 784 ± 203 | NA | NA | NA |
| Temperature (°C) | 48.1 ± 8.6 | 39.9 ± 15.3 | 18.7 ± 2.9 | 19.5 ± 2 | 19.8 ± 1.4 |
| Building with central hot water production (CB) | |||||
| Total Chlorine (mg/L) | 0.09 ± 0.07 | 0.05 ± 0.03 | 0.03 ± 0.02 | 0.06 ± 0.03 | 0.05 ± 0.04 |
| pH | 6.6 ± 1 | 6.7 ± 1.2 | NA | NA | NA |
| Conductivity (µS) | 791 ± 235 | 805 ± 254 | NA | NA | NA |
| Temperature (°C) | 48.3 ± 7.9 | 40.1 ± 7 | 17.4 ± 2.2 | 18.9 ± 2.1 | 16 ± 1.4 |
| Building with solar thermal system for hot water production (TB) | |||||
| Total Chlorine (mg/L) | 0 | 0 | 0 | 0 | 0 |
| pH | 6.9 ± 1 | 6.9 ± 1.1 | NA | NA | NA |
| Conductivity (µS) | 793 ± 215 | 799 ± 256 | NA | NA | NA |
| Temperature (°C) | 39.5 ± 8.9 | 33.8 ± 7.9 | 17.4 ± 2.6 | 18.9 ± 2.2 | 17.6 ± 2.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totaro, M.; Valentini, P.; Costa, A.L.; Frendo, L.; Cappello, A.; Casini, B.; Miccoli, M.; Privitera, G.; Baggiani, A. Presence of Legionella spp. in Hot Water Networks of Different Italian Residential Buildings: A Three-Year Survey. Int. J. Environ. Res. Public Health 2017, 14, 1296. https://doi.org/10.3390/ijerph14111296
Totaro M, Valentini P, Costa AL, Frendo L, Cappello A, Casini B, Miccoli M, Privitera G, Baggiani A. Presence of Legionella spp. in Hot Water Networks of Different Italian Residential Buildings: A Three-Year Survey. International Journal of Environmental Research and Public Health. 2017; 14(11):1296. https://doi.org/10.3390/ijerph14111296
Chicago/Turabian StyleTotaro, Michele, Paola Valentini, Anna Laura Costa, Lorenzo Frendo, Alessia Cappello, Beatrice Casini, Mario Miccoli, Gaetano Privitera, and Angelo Baggiani. 2017. "Presence of Legionella spp. in Hot Water Networks of Different Italian Residential Buildings: A Three-Year Survey" International Journal of Environmental Research and Public Health 14, no. 11: 1296. https://doi.org/10.3390/ijerph14111296
APA StyleTotaro, M., Valentini, P., Costa, A. L., Frendo, L., Cappello, A., Casini, B., Miccoli, M., Privitera, G., & Baggiani, A. (2017). Presence of Legionella spp. in Hot Water Networks of Different Italian Residential Buildings: A Three-Year Survey. International Journal of Environmental Research and Public Health, 14(11), 1296. https://doi.org/10.3390/ijerph14111296





