Prediction of Mortality in Patients with Isolated Traumatic Subarachnoid Hemorrhage Using a Decision Tree Classifier: A Retrospective Analysis Based on a Trauma Registry System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Decision Tree Classifier
2.3. Performance of the Decision Tree Classifier
2.4. Statistical Analysis
3. Results
3.1. Characteristics and Outcomes of Patients with Isolated tSAH
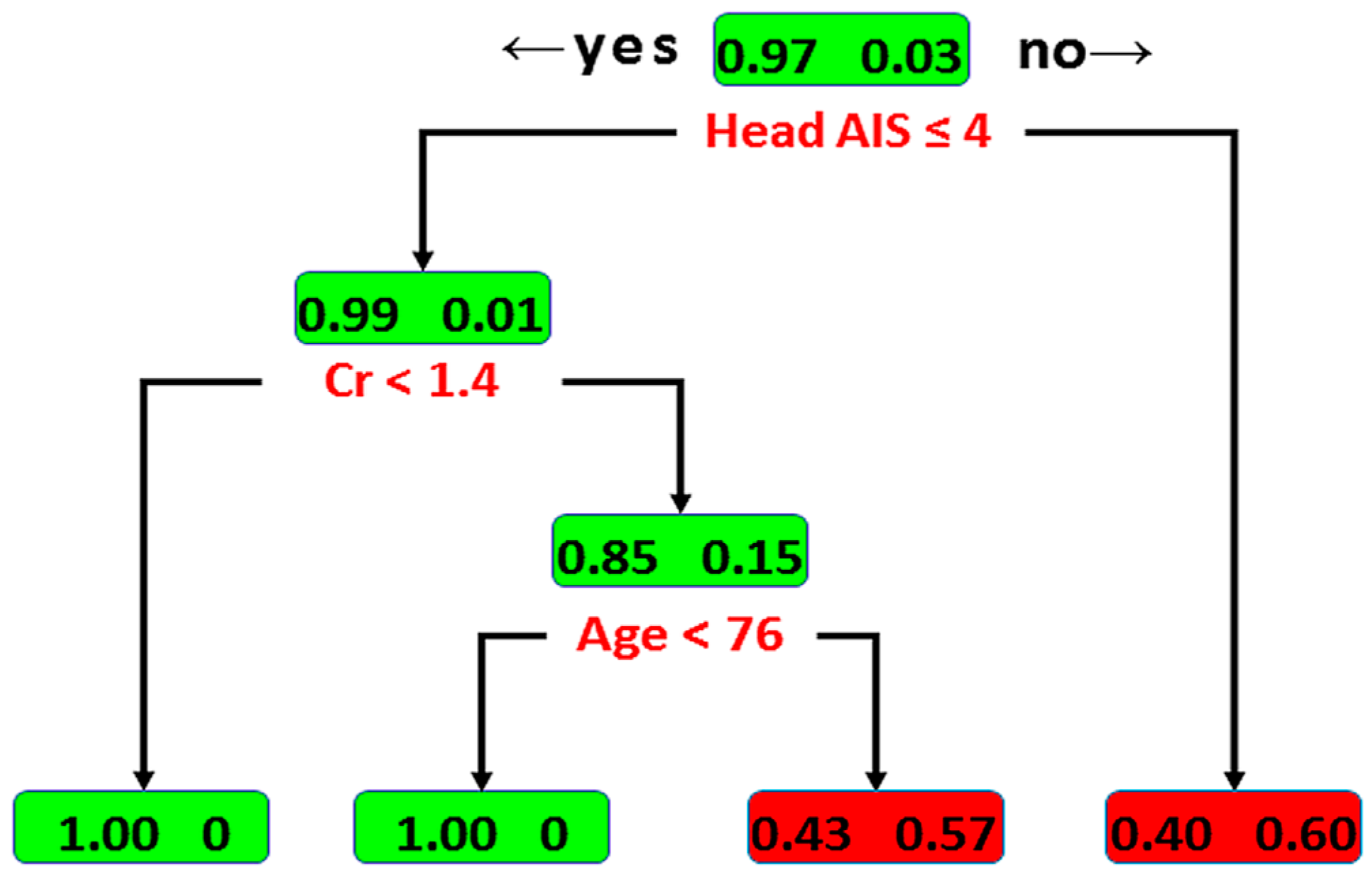
3.2. Classification by Decision Tree
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Servadei, F.; Murray, G.D.; Teasdale, G.M.; Dearden, M.; Iannotti, F.; Lapierre, F.; Maas, A.J.; Karimi, A.; Ohman, J.; Persson, L.; et al. Traumatic subarachnoid hemorrhage: Demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery 2002, 50, 261–267. [Google Scholar]
- Holmes, J.F.; Hendey, G.W.; Oman, J.A.; Norton, V.C.; Lazarenko, G.; Ross, S.E.; Hoffman, J.R.; Mower, W.R. Epidemiology of blunt head injury victims undergoing ED cranial computed tomographic scanning. Am. J. Emerg. Med. 2006, 24, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jakob, M.; Stapf, C.; Marshall, R.S.; Hartmann, A.; Mast, H. Multimodal early rehabilitation and predictors of outcome in survivors of severe traumatic brain injury. J. Trauma 2008, 65, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, C.; d’Avella, D.; Servadei, F.; Angileri, F.F.; Brambilla, G.; Conti, C.; Cristofori, L.; Delfini, R.; Denaro, L.; Ducati, A.; et al. Patients with moderate head injury: A prospective multicenter study of 315 patients. Neurosurgery 2009, 64, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Armin, S.S.; Colohan, A.R.; Zhang, J.H. Traumatic subarachnoid hemorrhage: Our current understanding and its evolution over the past half century. Neurol. Res. 2006, 28, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Parchani, A.; El-Menyar, A.; Al-Thani, H.; El-Faramawy, A.; Zarour, A.; Asim, M.; Latifi, R. Traumatic subarachnoid hemorrhage due to motor vehicle crash versus fall from height: A 4-year epidemiologic study. World Neurosurg. 2014, 82, e639–e644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Martin, N.A.; Alsina, G.; McArthur, D.L.; Zaucha, K.; Hovda, D.A.; Becker, D.P. Hemodynamically significant cerebral vasospasm and outcome after head injury: A prospective study. J. Neurosurg. 1997, 87, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Tsai, H.C.; Hsieh, T.C. The impact of traumatic subarachnoid hemorrhage on outcome: A study with grouping of traumatic subarachnoid hemorrhage and transcranial Doppler sonography. J. Trauma Acute Care Surg. 2012, 73, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.; Yeung, J.H.; Graham, C.A.; Zhu, X.L.; Rainer, T.H.; Poon, W.S. Neurological outcome in patients with traumatic brain injury and its relationship with computed tomography patterns of traumatic subarachnoid hemorrhage. J. Neurosurg. 2011, 114, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Badhiwala, J.H.; Witiw, C.D.; Mansouri, A.; Davidson, B.; Almenawer, S.A.; Lipsman, N.; Da Costa, L.; Pirouzmand, F.; Nathens, A.B. The clinical significance of isolated traumatic subarachnoid hemorrhage in mild traumatic brain injury: A meta-analysis. J. Trauma Acute Care Surg. 2017, 83, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.R.; Chew, B.G.; Swartz, C.E.; Wilberger, J.E. The clinical significance of isolated traumatic subarachnoid hemorrhage. J. Trauma Acute Care Surg. 2013, 74, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Phelan, H.A.; Richter, A.A.; Scott, W.W.; Pruitt, J.H.; Madden, C.J.; Rickert, K.L.; Wolf, S.E. Does isolated traumatic subarachnoid hemorrhage merit a lower intensity level of observation than other traumatic brain injury? J. Neurotrauma 2014, 31, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Segar, D.J.; Asaad, W.F. Comprehensive assessment of isolated traumatic subarachnoid hemorrhage. J. Neurotrauma 2014, 31, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Ditty, B.J.; Omar, N.B.; Foreman, P.M.; Patel, D.M.; Pritchard, P.R.; Okor, M.O. The nonsurgical nature of patients with subarachnoid or intraparenchymal hemorrhage associated with mild traumatic brain injury. J. Neurosurg. 2015, 123, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Borczuk, P.; Penn, J.; Peak, D.; Chang, Y. Patients with traumatic subarachnoid hemorrhage are at low risk for deterioration or neurosurgical intervention. J. Trauma Acute Care Surgery 2013, 74, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.; Mallory, G.; Planchard, R.; Nothdurft, G.; Graffeo, C.; Atkinson, J. Triage Patterns of Traumatic Subarachnoid Hemorrhage: Is Referral to a Tertiary Care Center Necessary? World Neurosurg. 2017, 100, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Farion, K.; Michalowski, W.; Wilk, S.; O’Sullivan, D.; Matwin, S. A tree-based decision model to support prediction of the severity of asthma exacerbations in children. J. Med. Syst. 2010, 34, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; King, N.K.; Neilson, S.J.; Gandhi, M.P.; Ng, I. External validation of the CRASH and IMPACT prognostic models in severe traumatic brain injury. J. Neurotrauma 2014, 31, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lingsma, H.F.; Steyerberg, E.W.; Maas, A.I. External Validation of the International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury: Prognostic Models for Traumatic Brain Injury on the Study of the Neuroprotective Activity of Progesterone in Severe Traumatic Brain Injuries Trial. J. Neurotrauma 2016, 33, 1535–1543. [Google Scholar] [PubMed]
- Perel, P.; Arango, M.; Clayton, T.; Edwards, P.; Komolafe, E.; Poccock, S.; Roberts, I.; Shakur, H.; Steyerberg, E.; Yutthakasemsunt, S. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429. [Google Scholar] [PubMed]
- Carter, E.L.; Hutchinson, P.J.; Kolias, A.G.; Menon, D.K. Predicting the outcome for individual patients with traumatic brain injury: A case-based review. Br. J. Neurosurg. 2016, 30, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.D.; Sutter, R. Prognosis in severe brain injury. Crit. Care Med. 2013, 41, 1104–1123. [Google Scholar] [CrossRef] [PubMed]
- Zintzaras, E.; Bai, M.; Douligeris, C.; Kowald, A.; Kanavaros, P. A tree-based decision rule for identifying profile groups of cases without predefined classes: Application in diffuse large B-cell lymphomas. Comput. Biol. Med. 2007, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Kasbekar, P.U.; Goel, P.; Jadhav, S.P. A Decision Tree Analysis of Diabetic Foot Amputation Risk in Indian Patients. Front. Endocrinol. 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Butcher, N.; Balogh, Z.J. AIS > 2 in at least two body regions: A potential new anatomical definition of polytrauma. Injury 2012, 43, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Wang, H.W. Analysis of traffic injury severity: An application of non-parametric classification tree techniques. Accid. Anal. Prev. 2006, 38, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B. Tree: Classification and Regression Trees. Available online: http://CRAN.R-project.org/package=tree (accessed on 27 July 2017).
- Guilbault, R.W.R.; Ohlsson, M.A.; Afonso, A.M.; Ebell, M.H. External Validation of Two Classification and Regression Tree Models to Predict the Outcome of Inpatient Cardiopulmonary Resuscitation. J. Viral Hepat. 2017, 32, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.Q.; Zhou, Y.Y.; Yan, H.D.; Li, H.; Wu, F.L.; Xie, Y.Y.; Braddock, M.; Lin, X.Y.; Zheng, M.H. Classification and regression tree analysis of acute-on-chronic hepatitis B liver failure: Seeing the forest for the trees. J. Viral Hepat. 2017, 24, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.K.; Balasubramani, G.K.; Nowalk, M.P.; Eng, H.; Urbanski, L.; Jackson, M.L.; Jackson, L.A.; McLean, H.Q.; Belongia, E.A.; Monto, A.S.; et al. Classification and Regression Tree (CART) analysis to predict influenza in primary care patients. BMC Infect. Dis. 2016, 16, 503. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.D.; Butcher, I.; McHugh, G.S.; Lu, J.; Mushkudiani, N.A.; Maas, A.I.; Marmarou, A.; Steyerberg, E.W. Multivariable prognostic analysis in traumatic brain injury: Results from the IMPACT study. J. Neurotrauma 2007, 24, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Alharfi, I.M.; Stewart, T.C.; Kelly, S.H.; Morrison, G.C.; Fraser, D.D. Hypernatremia is associated with increased risk of mortality in pediatric severe traumatic brain injury. J. Neurotrauma 2013, 30, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.; Sung, G.Y.; Straw, R.N.; Yahia, A.M.; Saad, M.; Guterman, L.R.; Hopkins, L.N. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2002, 50, 749–755. [Google Scholar] [CrossRef]
- Fisher, L.A.; Ko, N.; Miss, J.; Tung, P.P.; Kopelnik, A.; Banki, N.M.; Gardner, D.; Smith, W.S.; Lawton, M.T.; Zaroff, J.G. Hypernatremia predicts adverse cardiovascular and neurological outcomes after SAH. Neurocrit. Care 2006, 5, 180–185. [Google Scholar] [CrossRef]
- Takeuchi, J.; Yagi, S.; Nakayama, S.; Ikeda, T.; Uchida, E.; Inoue, G.; Shintani, F.; Ueda, H. Experimental studies on the nervous control of the renal circulation-Effect of electrical stimulation of the diencephalon on the renal circulation. Jpn. Heart J. 1960, 1, 288–299. [Google Scholar] [CrossRef]
- Neil-Dwyer, G.; Cruickshank, J.M.; Doshi, R. The stress response in subarachnoid haemorrhage and head injury. Acta Neurochir. Suppl. (Wien.) 1990, 47, 102–110. [Google Scholar] [PubMed]
- Mosenthal, A.C.; Lavery, R.F.; Addis, M.; Kaul, S.; Ross, S.; Marburger, R.; Deitch, E.A.; Livingston, D.H. Isolated traumatic brain injury: Age is an independent predictor of mortality and early outcome. J. Trauma 2002, 52, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Chieregato, A.; Fainardi, E.; Morselli-Labate, A.M.; Antonelli, V.; Compagnone, C.; Targa, L.; Kraus, J.; Servadei, F. Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery 2005, 56, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Stitzel, J.D.; Kilgo, P.D.; Danelson, K.A.; Geer, C.P.; Pranikoff, T.; Meredith, J.W. Age thresholds for increased mortality of three predominant crash induced head injuries. Ann. Adv. Automot. Med. 2008, 52, 235–244. [Google Scholar] [PubMed]
- Hayashi, T.; Karibe, H.; Narisawa, A.; Kameyama, M. Delayed Deterioration in Isolated Traumatic Subarachnoid Hemorrhage. World Neurosurg. 2016, 86, e9–e14. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Orringer, D.A.; Lau, D.; Fletcher, J.J. Cumulative incidence and predictors of neurosurgical interventions following nonsevere traumatic brain injury with mildly abnormal head imaging findings. J. Trauma Acute Care Surg. 2012, 73, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- The Abbreviated Injury Scale 1998 Revision; Association for the Advancement of Automotive Medicine: Barrington, IL, USA, 1998.
| Variables | Total (n = 545) | Survival (n = 533) | Mortality (n = 12) | p-Value | |
|---|---|---|---|---|---|
| Sex | Female | 270 (49.5%) | 264 (49.5%) | 6 (50%) | >0.999 |
| Male | 275 (50.5%) | 269 (50.5%) | 6 (50%) | ||
| CVA | No | 521 (95.6%) | 510 (95.7%) | 11 (91.7%) | 0.421 |
| Yes | 24(4.4%) | 23(4.3%) | 1 (8.3%) | ||
| CAD | No | 528 (96.9%) | 516 (96.8%) | 12 (100%) | >0.999 |
| Yes | 17 (3.1%) | 17 (3.2%) | 0 (0%) | ||
| HTN | No | 379 (69.5%) | 371 (69.6%) | 8 (66.7%) | 0.762 |
| Yes | 166(30.5%) | 162(30.4%) | 4 (33.3%) | ||
| CHF | No | 537(98.5%) | 525(98.5%) | 12 (100%) | >0.999 |
| Yes | 8(1.5%) | 8(1.5%) | 0(0%) | ||
| ESRD | No | 538 (98.7%) | 526 (98.7%) | 12 (100%) | >0.999 |
| Yes | 7(1.3%) | 7(1.3%) | 0(0%) | ||
| DM | No | 469 (86.1%) | 458 (85.9%) | 11 (91.7%) | >0.999 |
| Yes | 76 (13.9%) | 75 (14.1%) | 1 (8.3%) | ||
| AIS (Head) | 3 | 452 (82.9%) | 449 (84.2%) | 3 (25%) | <0.001 |
| 4 | 79 (14.5%) | 77 (14.5%) | 2 (16.7%) | ||
| 5 | 13 (2.4%) | 7 (1.3%) | 6 (50%) | ||
| 6 | 1 (0.2%) | 0 (0%) | 1 (8.3%) | ||
| AIS (Face) | 0 | 410 (75.2%) | 401 (75.2%) | 9 (75%) | >0.999 |
| 1 | 48 (8.8%) | 47 (8.9%) | 1 (8.3%) | ||
| 2 | 87 (16%) | 85 (15.9%) | 2 (16.7%) | ||
| AIS (Thorax) | 0 | 502 (92.1%) | 491 (92.1%) | 11 (91.7%) | 0.505 |
| 1 | 19 (3.5%) | 18 (3.4%) | 1 (8.3%) | ||
| 2 | 24 (4.4%) | 24 (4.5%) | 0 (0%) | ||
| AIS (Abdomen) | 0 | 534 (98%) | 523 (98.1%) | 11 (91.7%) | 0.007 |
| 1 | 4 (0.7%) | 3 (0.6%) | 1 (8.3%) | ||
| 2 | 7 (1.3%) | 7 (1.3%) | 0 (0%) | ||
| AIS (Extremity) | 0 | 390 (71.6%) | 381 (71.5%) | 9 (75%) | 0.965 |
| 1 | 52 (9.5%) | 51 (9.6%) | 1 (8.3%) | ||
| 2 | 103 (18.9%) | 101 (18.9%) | 2 (16.7%) | ||
| AIS (External) | 0 | 452 (82.9%) | 441 (82.7%) | 11 (91.7%) | 0.701 |
| 1 | 93 (17.1%) | 92 (17.3%) | 1 (8.3%) | ||
| Variables | Total (n = 545) | Survival (n = 533) | Mortality (n = 12) | p-Value |
|---|---|---|---|---|
| Age (years) | 58.0 (29.0) | 58 (28.5) | 72 (50.0) | 0.312 |
| BMI | 23.9 (3.9) | 23.9 (3.9) | 22.1 (5.7) | 0.149 |
| Shock index | 0.6 (0.2) | 0.6 (0.2) | 0.6 (0.4) | 0.183 |
| HR (beats/min) | 86 (21.0) | 86 (20.0) | 107 (23.3) | 0.018 |
| SBP (mmHg) | 149 (47.0) | 148 (46.5) | 167 (62.5) | 0.247 |
| RR (times/min) | 19 (2.0) | 19 (2.0) | 17.5 (5.5) | 0.077 |
| Temperature (°C) | 36.4 (0.7) | 36.4 (0.7) | 36.6 (0.8) | 0.740 |
| GCS | 15 (2.0) | 15 (2.0) | 3 (5.5) | <0.001 |
| ISS | 13 (5.0) | 11 (5.0) | 25 (26.3) | <0.001 |
| RBC (106/uL) | 4.4 (0.8) | 4.4 (0.8) | 4.5 (1.2) | 0.034 |
| WBC (103/uL) | 11.5 (6.8) | 11.5 (6.9) | 15.4 (10.2) | 0.304 |
| Hb (g/dL) | 13.2 (2.5) | 13.2 (2.5) | 13.2 (4.3) | 0.032 |
| Hct (%) | 39.3 (6.2) | 39.3 (6.3) | 39 (11.1) | 0.035 |
| Platelets (103/uL) | 207 (76.0) | 207 (77.0) | 209 (116.5) | 0.247 |
| Glucose (mg/dL) | 132 (49.0) | 131 (47.0) | 195.5 (271.8) | <0.001 |
| Na (mEq/L) | 139 (3.0) | 139 (3.0) | 139 (14.5) | 0.016 |
| K (mEq/L) | 3.6 (0.6) | 3.6 (0.6) | 3.8 (1.9) | 0.380 |
| BUN (mg/dL) | 13 (7.0) | 13 (7.0) | 18.5 (18.8) | 0.081 |
| Cr (mg/dL) | 0.8 (0.4) | 0.8 (0.4) | 1.1 (0.9) | 0.005 |
| AST (U/L) | 32 (19.0) | 32 (18.0) | 49 (74.0) | 0.003 |
| ALT (U/L) | 25 (18.0) | 25 (18.5) | 25.5 (29.8) | 0.202 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, C.-S.; Wu, S.-C.; Chien, P.-C.; Kuo, P.-J.; Chen, Y.-C.; Hsieh, H.-Y.; Hsieh, C.-H. Prediction of Mortality in Patients with Isolated Traumatic Subarachnoid Hemorrhage Using a Decision Tree Classifier: A Retrospective Analysis Based on a Trauma Registry System. Int. J. Environ. Res. Public Health 2017, 14, 1420. https://doi.org/10.3390/ijerph14111420
Rau C-S, Wu S-C, Chien P-C, Kuo P-J, Chen Y-C, Hsieh H-Y, Hsieh C-H. Prediction of Mortality in Patients with Isolated Traumatic Subarachnoid Hemorrhage Using a Decision Tree Classifier: A Retrospective Analysis Based on a Trauma Registry System. International Journal of Environmental Research and Public Health. 2017; 14(11):1420. https://doi.org/10.3390/ijerph14111420
Chicago/Turabian StyleRau, Cheng-Shyuan, Shao-Chun Wu, Peng-Chen Chien, Pao-Jen Kuo, Yi-Chun Chen, Hsiao-Yun Hsieh, and Ching-Hua Hsieh. 2017. "Prediction of Mortality in Patients with Isolated Traumatic Subarachnoid Hemorrhage Using a Decision Tree Classifier: A Retrospective Analysis Based on a Trauma Registry System" International Journal of Environmental Research and Public Health 14, no. 11: 1420. https://doi.org/10.3390/ijerph14111420






