The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
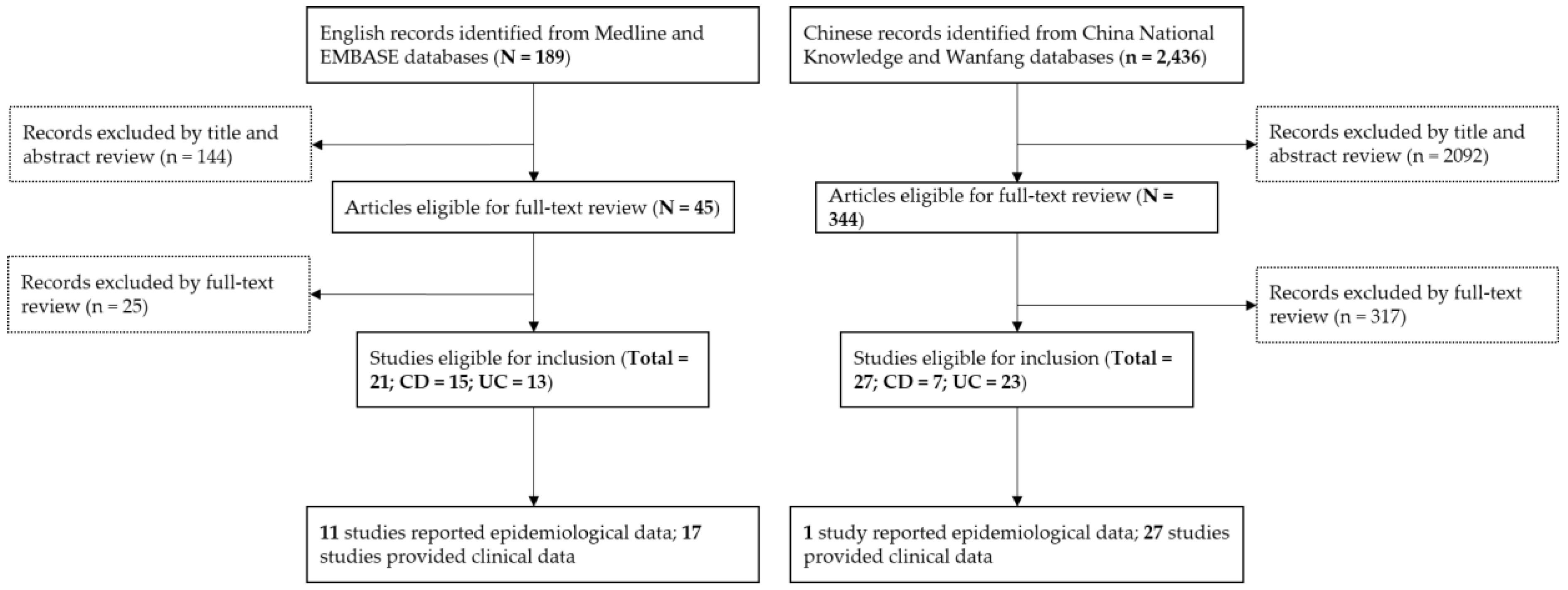
2.1. Literature Search and Selection Criteria
2.2. Data Extraction and Managemen
2.3. Data Analysis
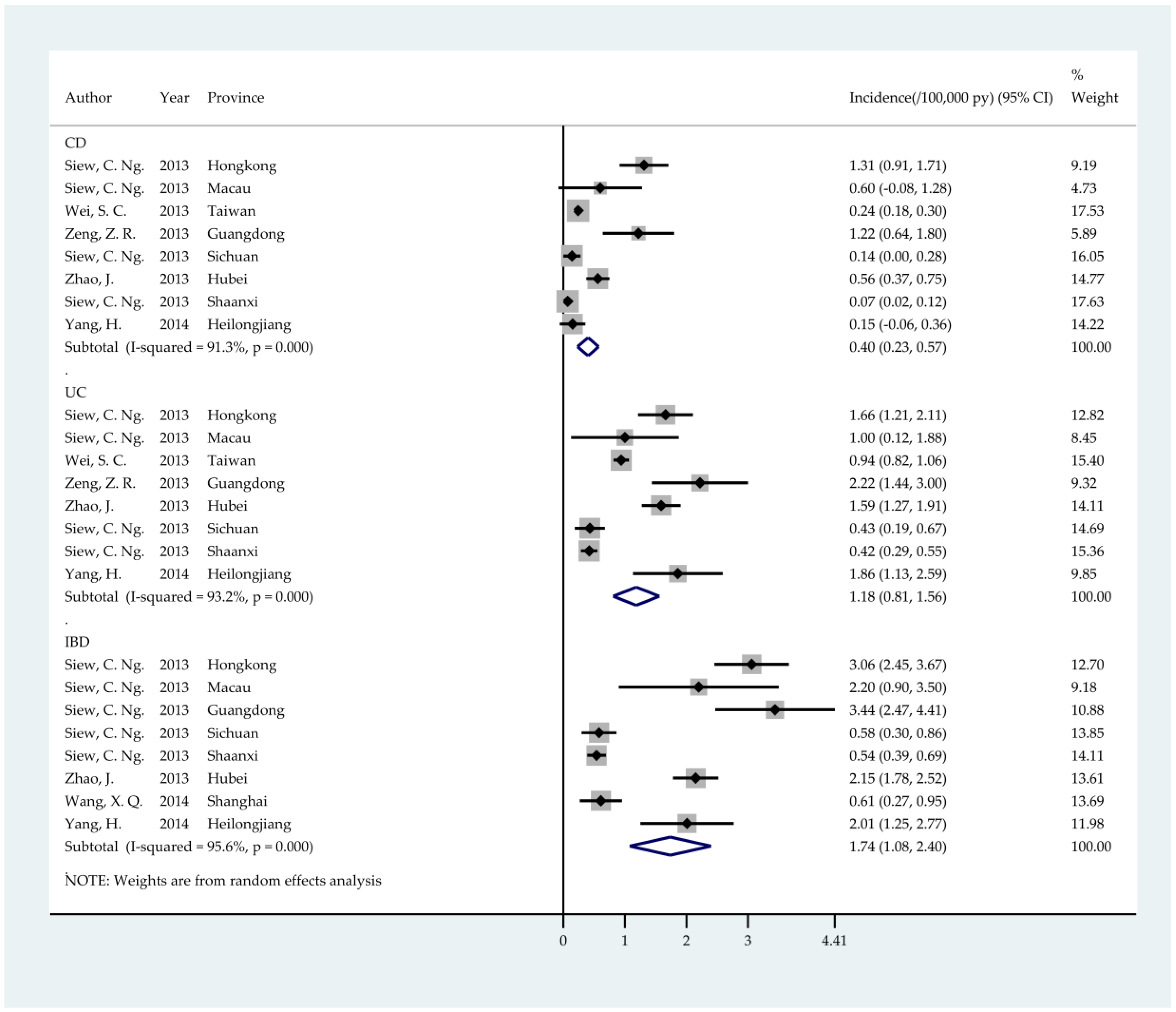
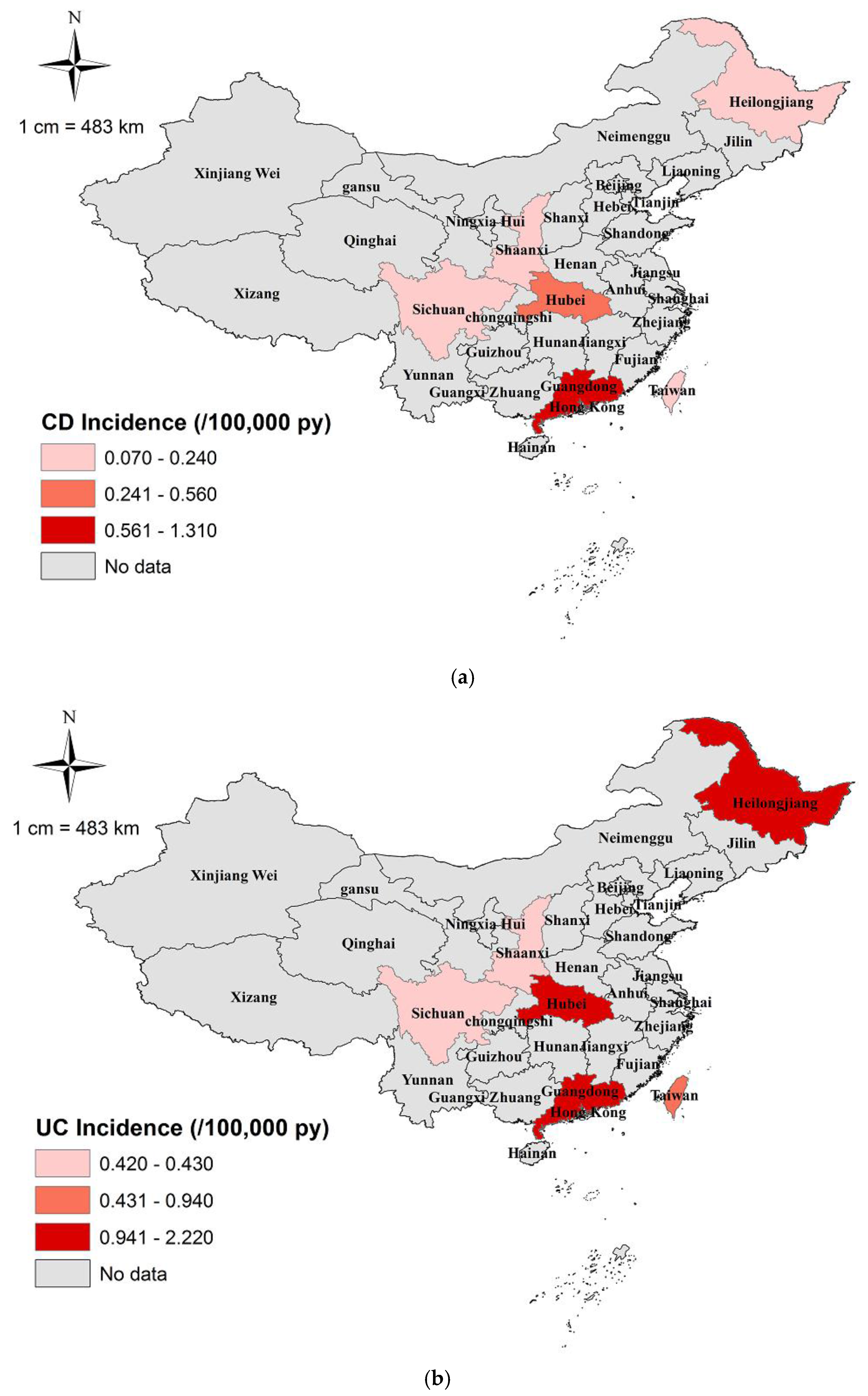
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Li, X.; Song, P.; Timofeeva, M.; Meng, X.; Rudan, I.; Little, J.; Satsangi, J.; Campbell, H.; Theodoratou, E. Systematic meta-analyses and field synopsis of genetic and epigenetic studies in paediatric inflammatory bowel disease. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Loftus, E.V., Jr.; Sandborn, W.J.; Yang, S.K. An update on the epidemiology of inflammatory bowel disease in Asia. Am. J. Gastroenterol. 2008, 103, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- APDW2004 Chinese IBD Working Group. Retrospective analysis of 515 cases of Crohn’s disease hospitalization in China: Nationwide study from 1990 to 2003. J. Gastroenterol. Hepatol. 2006, 21, 1009–1015. [Google Scholar]
- Wang, Y.; Ouyang, Q. Ulcerative colitis in China: Retrospective analysis of 3100 hospitalized patients. J. Gastroenterol. Hepatol. 2007, 22, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.J.; Zhu, X.S.; Huangfu, Z.; Shi, X.H.; Guo, Z.R. Prevalence and incidence rates of Crohn’s disease in mainland China: A meta-analysis of 55 years of research. J. Dig. Dis. 2010, 11, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; Swart, R.; Jordaan, E.; Mazinu, M.; Watermeyer, G. The association between race and Crohn’s disease phenotype in the Western Cape population of South Africa, defined by the Montreal Classification System. PloS ONE 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, R.K.; Jung, C.; Wasan, S.; Joshi, G.; Sawyer, R.; Roghmann, M.C. Racial differences in disease phenotypes in patients with Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Wexner, S.D.; Liu, Q.S.; Li, L.; Weiss, E.; Zhao, R.H. The differences between American and Chinese patients with Crohn’s disease. Colorectal Dis. Offi. J. Assoc. Coloproctol. G. B. Irel. 2011, 13, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, W.H.; Cheon, J.H. Clinical characteristics and treatment of inflammatory bowel disease: A comparison of eastern and western perspectives. World J. Gastroenterol. 2014, 20, 11525–11537. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a working party of the 2005 Montreal world congress of gastroenterology. Can. J. Gastroenterol. 2005, 19, 5a–36a. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, G.C.; Chong, C.A.; Chong, R.Y. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J. Crohn’s Colitis 2014, 8, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Loftus, E.V., Jr.; Sandborn, W.J. Epidemiology of inflammatory bowel disease in Asia. Inflamm. Bowel Dis. 2001, 7, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Zelinkova, Z.; van der Woude, C.J. Gender and inflammatory bowel disease. J. Clin. Cell. Immunol. 2014. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Swarbrick, E.T.; Irvine, E.J. A critical review of epidemiological studies in inflammatory bowel disease. Scand. J. Gastroenterol. 2001, 36, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Ishige, T.; Tomomasa, T.; Takebayashi, T.; Asakura, K.; Watanabe, M.; Suzuki, T.; Miyazawa, R.; Arakawa, H. Inflammatory bowel disease in children: Epidemiological analysis of the nationwide IBD registry in Japan. J. Gastroenterol. 2010, 45, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Song, S.M.; Kim, K.M.; Lee, Y.J.; Rhee, K.W.; Jang, J.Y.; Park, S.J.; Yoon, C.H. Characteristics and trends in the incidence of inflammatory bowel disease in Korean children: A single-center experience. Dig. Dis. Sci. 2010, 55, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Pedersen, N.; Cukovic-Cavka, S.; Brinar, M.; Kaimakliotis, I.; Duricova, D.; Shonova, O.; Vind, I.; Avnstrom, S.; Thorsgaard, N.; et al. East-west gradient in the incidence of inflammatory bowel disease in europe: The ECCO-EpiCom inception cohort. Gut 2014, 63, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivananda, S.; Lennard-Jones, J.; Logan, R.; Fear, N.; Price, A.; Carpenter, L.; van Blankenstein, M. Incidence of inflammatory bowel disease across Europe: Is there a difference between north and south? Results of the European collaborative study on inflammatory bowel disease (EC-IBD). Gut 1996, 39, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nature reviews. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar]
- Giovino, G.A.; Mirza, S.A.; Samet, J.M.; Gupta, P.C.; Jarvis, M.J.; Bhala, N.; Peto, R.; Zatonski, W.; Hsia, J.; Morton, J.; et al. Tobacco use in 3 billion individuals from 16 countries: An analysis of nationally representative cross-sectional household surveys. Lancet 2012, 380, 668–679. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Prescott, N.J.; Pessoa-Lopes, P.; Mathew, C.G.; Sanderson, J.; Hart, A.L.; et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm. Bowel Dis. 2012, 18, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Lunney, P.C.; Leong, R.W. Review article: Ulcerative colitis, smoking and nicotine therapy. Aliment. Pharmacol. Therapeut. 2012, 36, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Kulkarni, G.; Shen, B. Menstrual cycle, sex hormones in female inflammatory bowel disease patients with and without surgery. J. Dig. Dis. 2015, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, J.; Baldassano, R.N. Inflammatory bowel disease: The difference between children and adults. Inflamm. Bowel Dis. 2008, 14, S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Collard, A.; Oger, A.F.; Degroote, E.; Aboul Nasr El Yafi, F.A.; Belaiche, J. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001, 49, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Solberg, I.C.; Vatn, M.H.; Hoie, O.; Stray, N.; Sauar, J.; Jahnsen, J.; Moum, B.; Lygren, I. Clinical course in Crohn’s disease: Results of a Norwegian population-based ten-year follow-up study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2007, 5, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, L.; Pandur, T.; David, G.; Balogh, Z.; Kuronya, P.; Tollas, A.; Lakatos, P.L. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: Results of a 25-year follow-up study. World J. Gastroenterol. 2003, 9, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Prinz Vavricka, B.M.; Zeitz, J.; Rogler, G.; Schoepfer, A.M. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 2011, 106, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.M. Specificities of inflammatory bowel disease in childhood. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Sawczenko, A.; Sandhu, B. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch. Dis. Child. 2003, 88, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Langholz, E.; Munkholm, P.; Davidsen, M.; Binder, V. Course of ulcerative colitis: Analysis of changes in disease activity over years. Gastroenterology 1994, 107, 3–11. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, H.; Ouyang, Q. Clinical manifestations of inflammatory bowel disease: East and west differences. J. Dig. Dis. 2007, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Sauar, J.; Kjellevold, O.; Schulz, T.; Vatn, M.H.; Moum, B. Ulcerative colitis and clinical course: Results of a 5-year population-based follow-up study (the IBSEN study). Inflamm. Bowel Dis. 2006, 12, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.L.; Lana, R.; Taxonera, C.; Alba, C.; Izquierdo, S.; Diaz-Rubio, M. Extraintestinal manifestations in inflammatory bowel disease: Differences between Crohn’s disease and ulcerative colitis. Med. Clin. 2005, 125, 297–300. [Google Scholar]
- Williams, H.; Walker, D.; Orchard, T.R. Extraintestinal manifestations of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2008, 10, 597–605. [Google Scholar] [CrossRef] [PubMed]

| CD Phenotype | No. of Cases | Proportion (%) | 95% CI |
|---|---|---|---|
| Age at diagnosis (n = 1464) | |||
| A1 (≤16 years) | 204 | 13.9 | 12.2 to 15.7 |
| A2 (17–40 years) | 940 | 64.2 | 61.8 to 66.7 |
| A3 (<40 years) | 320 | 21.9 | 19.7 to 24.0 |
| Location (n = 2153) | |||
| L1 (Terminal ileum) | 652 | 30.3 | 28.3 to 32.2 |
| L2 (Colon) | 671 | 31.2 | 29.2 to 33.1 |
| L3 (Ileocolon) | 763 | 35.4 | 33.4 to 37.5 |
| L4 (Upper GI) | 47 | 2.2 | 1.6 to 2.8 |
| L1 + L4 (Terminal ileum + Upper GI) | 8 | 0.4 | 0.1 to 0.6 |
| L2 + L4 (Colon + Upper GI) | 1 | 0.1 | 0.0 to 0.2 |
| L3 + L4 (Ileocolon + Upper GI) | 11 | 0.5 | 0.2 to 0.8 |
| Behaviour (n = 1221) | |||
| B1 (Non-stricturing + non-penetrating) | 538 | 44.1 | 41.3 to 46.9 |
| B2 (Stricturing) | 355 | 29.0 | 26.5 to 31.6 |
| B3 (Penetrating) | 234 | 19.2 | 17.0 to 21.4 |
| B1p (Non-stricturing + non-penetrating + perianal) | 59 | 4.8 | 3.6 to 6.0 |
| B2p (Stricturing + perianal) | 10 | 0.8 | 0.3 to 1.3 |
| B3p (Penetrating + perianal) | 25 | 2.1 | 1.3 to 2.8 |
| Clinical symptoms (n = 924) | |||
| Abdominal pain | 734 | 79.4 | 76.8 to 82.1 |
| Diarrhoea | 501 | 54.2 | 51.0 to 57.4 |
| Weight loss | 410 | 44.4 | 41.2 to 47.6 |
| Fever | 296 | 32.0 | 29.0 to 35.0 |
| Anaemia | 229 | 24.8 | 22.0 to 27.6 |
| Bloody stool | 148 | 16.0 | 13.7 to 18.4 |
| Haemorrhage | 139 | 15.0 | 12.7 to 17.3 |
| Constipation | 71 | 7.7 | 6.0 to 9.4 |
| Extra-intestinal manifestations (n = 979) | |||
| Joint | 69 | 7.1 | 5.5 to 8.7 |
| Mouth | 56 | 5.8 | 4.3 to 7.2 |
| Skin | 28 | 2.9 | 1.8 to 3.9 |
| Biliary | 25 | 2.6 | 1.6 to 3.5 |
| Eyes | 16 | 1.6 | 0.8 to 2.4 |
| Others | 25 | 2.6 | 1.6 to 3.5 |
| UC Phenotype | No. of Cases | Proportion (%) | 95% CI |
|---|---|---|---|
| Age at diagnosis (N = 8895) | |||
| A1 (≤16 years) | 190 | 2.1 | 1.8 to 2.4 |
| A2 (17–40 years) | 3025 | 34.0 | 33.0 to 35.0 |
| A3 (<40 years) | 5680 | 63.9 | 62.9 to 64.9 |
| Location (n = 17,371) | |||
| Proctitis | 4643 | 26.7 | 26.1 to 27.4 |
| Proctosigmoiditis | 8384 | 48.3 | 47.5 to 49.0 |
| Pancolitis | 2798 | 16.1 | 15.6 to 16.7 |
| Extensive colitis | 1546 | 8.9 | 8.4 to 9.3 |
| Severity (n = 11,389) | |||
| Mild | 3447 | 30.3 | 29.4 to 31.1 |
| Moderate | 5268 | 46.3 | 45.3 to 47.2 |
| Severe | 2674 | 23.5 | 22.7 to 24.3 |
| Clinical symptoms (n = 11,305) | |||
| Diarrhoea | 9457 | 83.7 | 83.0 to 84.3 |
| Abdominal pain | 7340 | 64.9 | 64.1 to 65.8 |
| Bloody stool | 5997 | 53.1 | 52.1 to 54.0 |
| Weight loss | 3215 | 28.4 | 27.7 to 29.3 |
| Mucus | 3254 | 28.8 | 28.0 to 29.6 |
| Fever | 2054 | 18.2 | 17.5 to 18.9 |
| Anaemia | 1512 | 13.4 | 12.8 to 14.0 |
| Constipation | 654 | 5.8 | 5.4 to 6.2 |
| Haemorrhage | 257 | 2.3 | 2.0 to 2.6 |
| Extra-intestinal manifestations (n = 6886) | |||
| Joint | 368 | 5.4 | 4.8 to 5.9 |
| Mouth | 146 | 2.1 | 1.8 to 2.5 |
| Skin | 101 | 1.5 | 1.2 to 1.8 |
| Eyes | 68 | 1.0 | 0.8 to 1.2 |
| Biliary | 4 | 0.1 | 0.0 to 0.1 |
| Others | 65 | 1.0 | 0.7 to 1.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Song, P.; Li, J.; Tao, Y.; Li, G.; Li, X.; Yu, Z. The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2017, 14, 238. https://doi.org/10.3390/ijerph14030238
Li X, Song P, Li J, Tao Y, Li G, Li X, Yu Z. The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2017; 14(3):238. https://doi.org/10.3390/ijerph14030238
Chicago/Turabian StyleLi, Xue, Peige Song, Jun Li, Yuchang Tao, Guowei Li, Xiumin Li, and Zengli Yu. 2017. "The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 14, no. 3: 238. https://doi.org/10.3390/ijerph14030238
APA StyleLi, X., Song, P., Li, J., Tao, Y., Li, G., Li, X., & Yu, Z. (2017). The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 14(3), 238. https://doi.org/10.3390/ijerph14030238





