Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Populations, Case Definitions and Categorization
2.3. Definitions of Exposure
2.4. Epidemiologic Investigations
2.5. Laboratory Tests
2.6. Data Statistical Analysis
3. Results
3.1. Epidemiological Findings in the HPAI (H5N1 and H5N6) and LPAI (H7N9 and H9N2) Fatalities and Survivors in the Overall Population
3.1.1. Overall Case Fatality Rate (CFR)
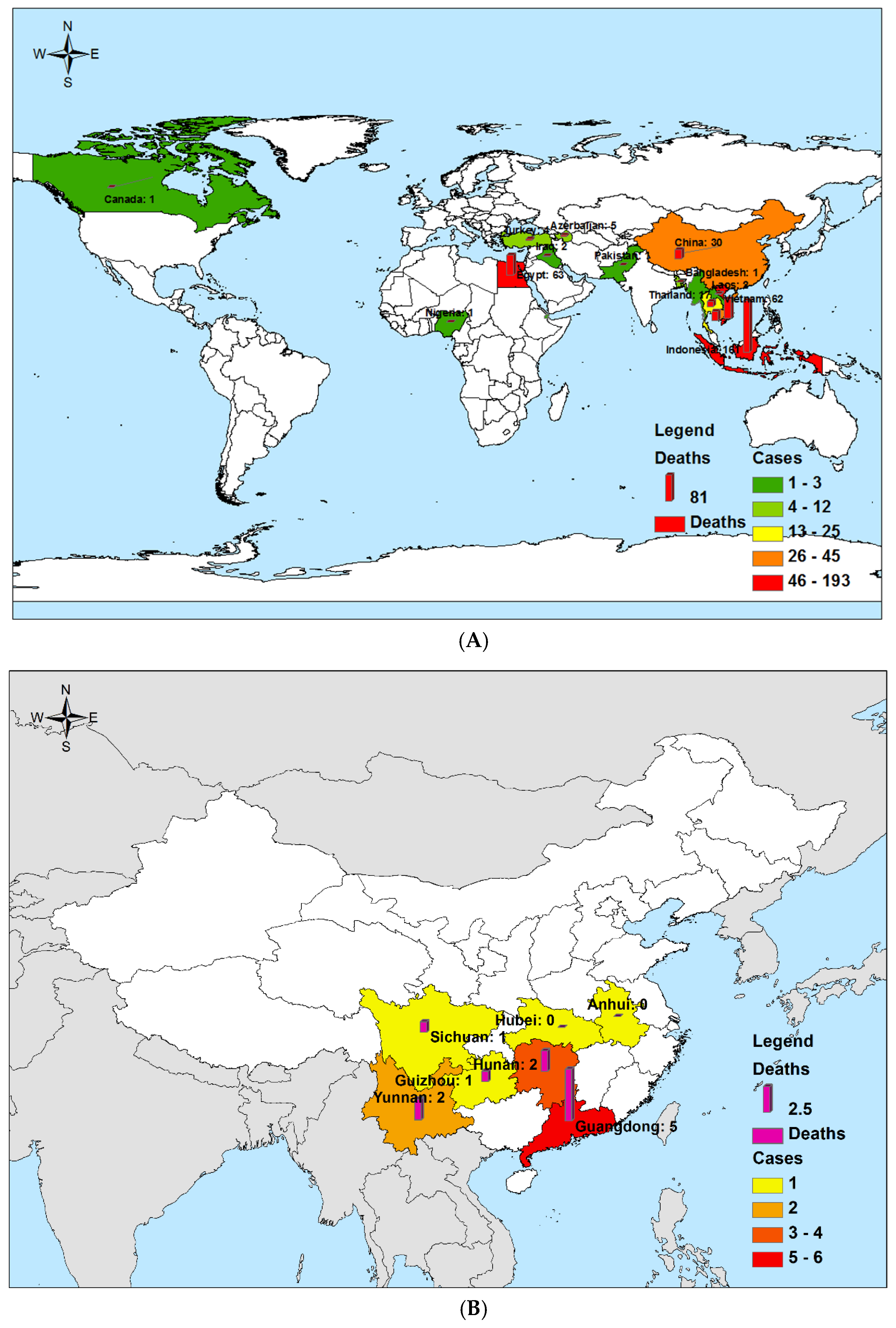
3.1.2. Diseases Distribution
3.1.3. Exposure History
3.2. Clinical Findings in the HPAI (H5N1 and H5N6) and LPAI (H7N9 and H9N2) Fatalities and Survivors in the Overall Population
3.2.1. Comorbidity
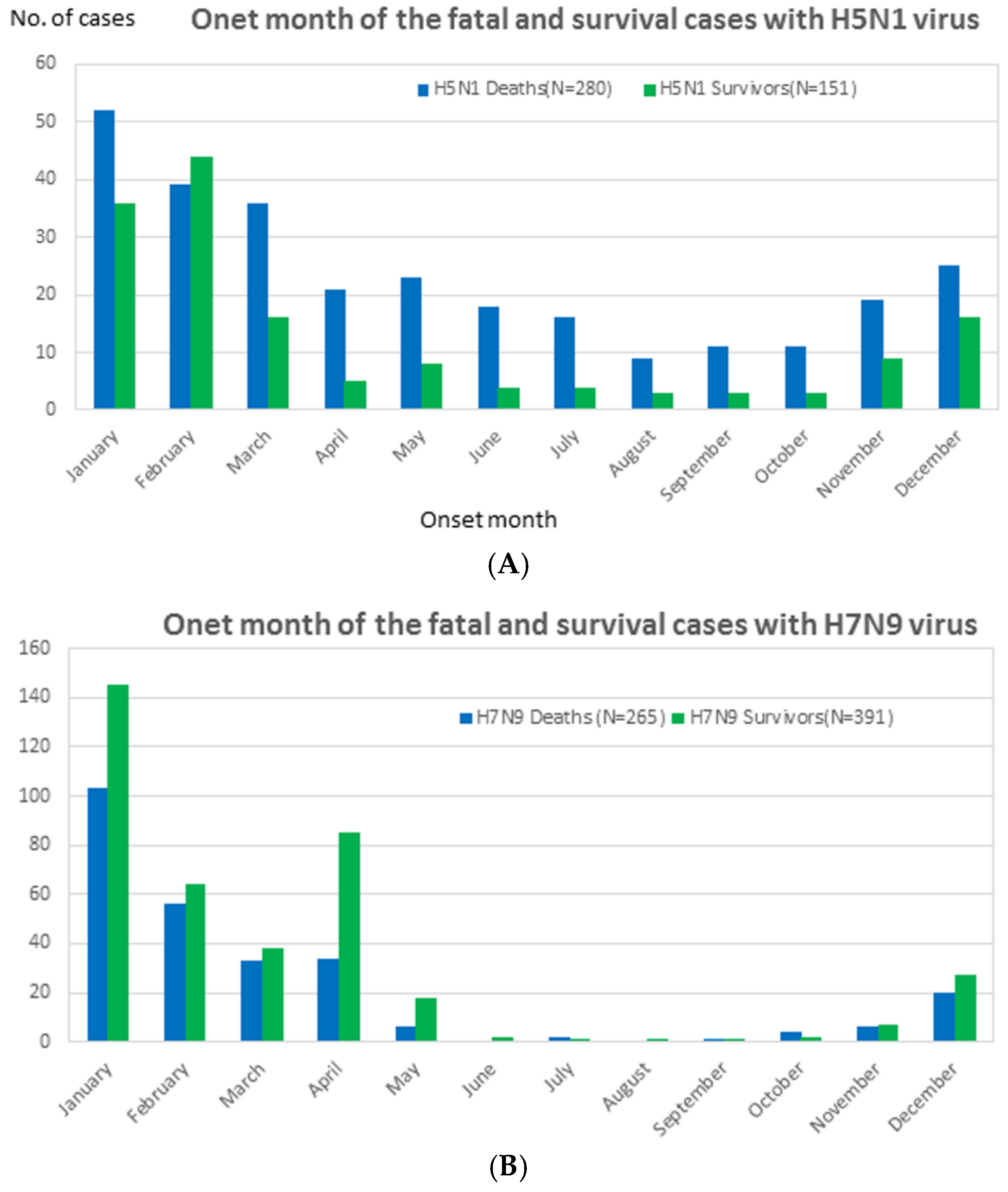
3.2.2. The Clinical Period
3.3. Comparative Epidemiology of the Fatalities and Survivors of HPAI (H5N1) and LPAI (H7N9) in Children (<15 Years Old)
3.3.1. CFR in Children
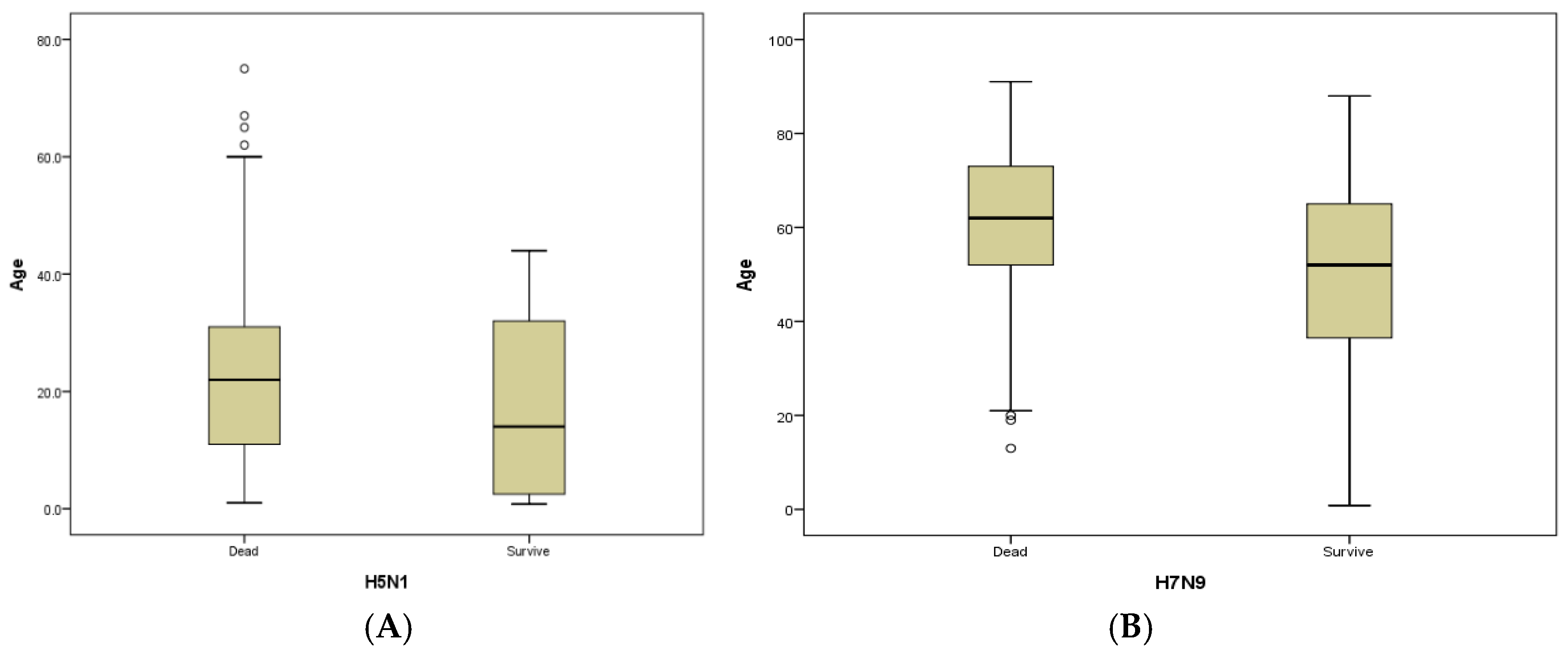
3.3.2. Age and Gender Distribution
3.4. Clinical Findings of the Fatalities and Survivors of HPAI (H5N1) and LPAI (H7N9) in Children (<15 years old)
3.5. Comparative Epidemiology of the Index and Secondary Deaths in the Clustered HPAI (H5N1) and LPAI (H7N9) Cases
3.5.1. CFR in the Clustered Cases
3.5.2. Exposure History
3.6. Multivariate Logistic Regression Model Assessing Odds Ratios of Risk for Death for Case-Patients Infected with Highly Pathogenic Avian Influenza (H5N1) and Low Pathogenicity Avian Influenza (H7N9) Virus
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CFR | Case-fatality rate. |
| ILI | Influenza-like illness. |
| rRT-PCR | Reverse transcription polymerase chain reaction. |
| HPAI | Highly pathogenic avian influenza. |
| LBM | Live bird markets. |
| LPAI | Low pathogenicity avian influenza virus. |
References
- Jernigan, D.B.; Cox, N.J. H7N9: Preparing for the unexpected in influenza. Annu. Rev. Med. 2015, 66, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Senne, D.A. Avian influenza in North and South America, 2002–2005. Avian. Dis. 2007, 51, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; Swayne, D.E.; Suarez, D.L.; Senne, D.A.; Pedersen, J.C.; Killian, M.L.; Pasick, J.; Handel, K.; Pillai, S.P.; Lee, C.W.; et al. Characterization of low-pathogenicity H5N1 avian influenza viruses from North America. J. Virol. 2007, 81, 11612–11619. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.A.; Arsnoe, D.M.; Minnis, R.B.; King, D.T.; Swafford, S.; Pedersen, K.; Owen, J.C. Prevalence of avian paramyxovirus 1 and avian influenza virus in double-crested Cormorants (Phalacrocorax auritus) in eastern North America. J. Wildl. Dis 2013, 49, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Cai, J.; Miao, Z.; Lu, M.; Qin, S.; Wang, X.; Lv, H.; Yu, Z.; Amer, S.; et al. Epidemiological, clinical and viral characteristics of fatal cases of human avian influenza A (H7N9) virus in Zhejiang Province, China. J. Infect. 2013, 67, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, L.; Zhou, M.; Chen, Z.; Li, F.; Wu, H.; Xiang, N.; Chen, E.; Tang, F.; Wang, D.; et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N. Engl. J. Med. 2014, 370, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Flint, P.L.; Pearce, J.M.; Franson, J.C.; Derksen, D.V. Wild bird surveillance for highly pathogenic avian influenza H5 in North America. Virol. J. 2015, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Jin, L.; Lau, E.H.; Liao, Q.; Wu, P.; Jiang, H.; Tsang, T.K.; Zheng, J.; Fang, V.J.; Chang, Z.; et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: A population-based study of laboratory-confirmed cases. Lancet 2013, 382, 129–137. [Google Scholar] [CrossRef]
- Liu, T.; Bi, Z.; Wang, X.; Li, Z.; Ding, S.; Bi, Z.; Wang, L.; Pei, Y.; Song, S.; Zhang, S.; et al. One family cluster of avian influenza A (H7N9) virus infection in Shandong, China. BMC Infect. Dis. 2014, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Horby, P.W.; Tsang, T.K.; Chen, E.; Gao, L.; Ou, J.; Nguyen, T.H.; Duong, T.N.; Gasimov, V.; Feng, L.; et al. Differences in the Epidemiology of Human Cases of Avian Influenza A(H7N9) and A(H5N1) Viruses Infection. Clin. Infect. Dis. 2015, 61, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Z.; Ma, C.; Jiang, C.; Feng, C.; Shanker, N.; Yang, P.; Sun, W.; Wang, Q. Cluster of human infections with avian influenza A (H7N9) cases: A temporal and spatial analysis. Int. J. Environ. Res. Public Health 2015, 12, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.; Liu, W.; Zeng, T.; Liu, Y.; Zhang, L.; Khan, A.; Wu, X.; Wu, R.; Wu, S.; Huang, L.; et al. Probable Hospital Cluster of H7N9 Influenza Infection. N. Engl. J. Med. 2016, 374, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bi, Y.; Tianm, H.; Li, X.; Liu, D.; Wu, Y.; Jin, T.; Wang, Y.; Chen, Q.; Chen, Z.; et al. Human infection with influenza virus A(H10N8) from live poultry markets, China, 2014. Emerg. Infect. Dis. 2014, 20, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wu, B.; Chen, Y.; Bi, Y.; Xie, Q. Influenza A(H5N6) Virus Reassortant, Southern China, 2014. Emerg. Infect. Dis. 2015, 21, 1261–1262. [Google Scholar] [CrossRef] [PubMed]
- CDC. Influenza Type A Viruses. 2015. Available online: https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm (accessed on 9 February 2015). [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062. [Google Scholar]
- Liu, S.L.; Wang, J.; Yang, X.H.; Chen, J.; Huang, R.J.; Ruan, B.; He, H.X.; Wang, C.M.; Zhang, H.M.; Sun, Z.; et al. Pandemic influenza A(H1N1) 2009 virus in pregnancy. Rev. Med. Virol. 2013, 23, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Chong, Y.H.; Katz, J.M.; Lim, W.; Ho, Y.Y.; Wang, S.S.; Tsang, T.H.; Au, W.W.; Chan, S.C.; Rowe, T.; et al. Lack of evidence for human-to-human transmission of avian influenza A (H9N2) viruses in Hong Kong, China 1999. Emerg. Infect. Dis. 2002, 8, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Sha, J.P.; Yu, Z.; Zhao, N.; Cheng, W.; Chan, T.C.; Amer, S.; Zhang, Z.R.; Liu, S.L. Epidemiological and virological differences in human clustered and sporadic infections with avian influenza A H7N9. Int. J. Infect. Dis. 2016, 49, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.C.; Li, K.B.; Chen, Z.Q.; Di, B.; Yang, Z.C.; Yuan, J.; Luo, H.B.; Ye, S.L.; Liu, H.; Lu, J.Y.; et al. Transmission of avian influenza A(H7N9) virus from father to child: A report of limited person-to-person transmission, Guangzhou, China, January 2014. Euro. Surveill. 2014, 19, 25. [Google Scholar] [CrossRef]
- Yu, H.; Shu, Y.; Hu, S.; Zhang, H.; Gao, Z.; Chen, H.; Dong, J.; Xu, C.; Zhang, Y.; Xiang, N.; et al. The first confirmed human case of avian influenza A (H5N1) in Mainland China. Lancet 2006, 367, 84. [Google Scholar] [CrossRef]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Mok, C.K.; Peiris, J.S.; Zhong, N.S. Human Infection with a Novel Avian Influenza A (H5N6) Virus. N. Engl. J. Med. 2015, 373, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Havers, F.; Chen, E.; Yuan, Z.; Yuan, H.; Ou, J.; Shang, M.; Kang, K.; Liao, K.; Liu, F.; et al. Risk factors for influenza A(H7N9) disease—China, 2013. Clin. Infect. Dis. 2014, 59, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Alraddadi, B.; Qutub, M.; Shabouni, O.; Curns, A.; Oboho, I.K.; Tomczyk, S.M.; Wolff, B.; Watson, J.T.; Madani, T.A. Association of Higher MERS-CoV Virus Load with Severe Disease and Death, Saudi Arabia, 2014. Emerg. Infect. Dis. 2015, 21, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Yang, Y.; Sun, Y.; Chen, W.J.; Sun, R.X.; Liu, K.; Ma, M.J.; Liang, S.; Yao, H.W.; Gray, G.C.; et al. Risk Distribution of Human Infections with Avian Influenza H7N9 and H5N1 virus in China. Sci. Rep. 2015, 5, 18610. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.S.; Kluberg, S.A.; Mekaru, S.R.; Brownstein, J.S. Mortality Risk Factors for Middle East Respiratory Syndrome Outbreak, South Korea, 2015. Emerg. Infect. Dis. 2015, 21, 2088–2090. [Google Scholar] [CrossRef] [PubMed]
- Alraddadi, B.M.; Watson, J.T.; Almarashi, A.; Abedi, G.R.; Turkistani, A.; Sadran, M.; Housa, A.; Almazroa, M.A.; Alraihan, N.; Banjar, A.; et al. Risk Factors for Primary Middle East Respiratory Syndrome Coronavirus Illness in Humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghafar, A.N.; Chotpitayasunondh, T.; Gao, Z.; Hayden, F.G.; Nguyen, D.H.; de Jong, M.D.; Naghdaliyev, A.; Peiris, J.S.; Shindo, N.; Soeroso, S.; et al. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 2008, 358, 261–273. [Google Scholar] [PubMed]
- Wang, X.; Fang, S.; Lu, X.; Xu, C.; Cowling, B.J.; Tang, X.; Peng, B.; Wu, W.; He, J.; Tang, Y.; et al. Seroprevalence to avian influenza A (H7N9) virus among poultry workers and the general population in southern China: A longitudinal study. Clin. Infect. Dis. 2014, 59, e76–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, Y.; Cui, D.; Yao, H.; Lou, J.; Huo, Z.; Xie, G.; Yu, F.; Zheng, S.; Yang, Y.; et al. Avian-origin influenza A(H7N9) infection in influenza A(H7N9)-affected areas of China: a serological study. J. Infect. Dis. 2014, 209, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; He, Y.; Wu, H.; Gao, X.; Pan, Q.; Shen, J.; Zhu, J.; Chen, H.; Zhu, Y.; et al. Mild infection of a novel H7N9 avian influenza virus in children in Shanghai. Emerg. Microbes. Infect. 2013, 2, e41. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Mai, W.; Shu, B.; Yi, L.; Lu, J.; Song, T.; Zhong, H.; Xiao, H.; Guan, D.; Wu, J.; et al. Mild influenza A/H7N9 infection among children in Guangdong Province. Pediatr. Infect. Dis. J. 2015, 34, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Ip, D.K.; Liao, Q.; Wu, P.; Gao, Z.; Cao, B.; Feng, L.; Xu, X.; Jiang, H.; Li, M.; Bao, J.; et al. Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: Case series. BMJ 2013, 346, f3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.V. The clinical presentation and outcomes of children infected with newly identified respiratory tract viruses. Infect. Dis. Clin. N. Am. 2005, 19, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cowling, B.J.; Feng, L.; Lau, E.H.; Liao, Q.; Tsang, T.K.; Peng, Z.; Wu, P.; Liu, F.; Fang, V.J.; et al. Human infection with avian influenza A H7N9 virus: An assessment of clinical severity. Lancet 2013, 382, 138–145. [Google Scholar] [CrossRef]
- Simonsen, L.; Taylor, R.J.; Young-Xu, Y.; Haber, M.; May, L.; Klugman, K.P. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio 2011, 2, e00309–e00310. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Peng, Z.; Fang, V.J.; Feng, L.; Tsang, T.K.; Jiang, H.; Lau, E.H.; Yang, J.; Zheng, J.; Qin, Y.; et al. Human Infection with Influenza A (H7N9) Virus during 3 Major Epidemic Waves, China, 2013–2015. Emerg. Infect. Dis. 2016, 22, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, W.; Yang, S.; Wu, N.; Gao, H.; Sheng, J.; Yao, H.; Wo, J.; Fang, Q.; Cui, D.; et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 2013, 381, 1916–1925. [Google Scholar] [CrossRef]
- Shen, Y.; Lu, H.; Qi, T.; Gu, Y.; Xiang, M.; Lu, S.; Qu, H.; Zhang, W.; He, J.; Cao, H.; et al. Fatal cases of human infection with avian influenza A (H7N9) virus in Shanghai, China in 2013. Biosci. Trends 2015, 9, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Adisasmito, W.; Chan, P.K.; Lee, N.; Oner, A.F.; Gasimov, V.; Aghayev, F.; Zaman, M.; Bamgboye, E.; Dogan, N.; Coker, R.; et al. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: Analysis of a Global Patient Registry. J. Infect. Dis. 2010, 202, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.K.; Yang, S.; Acosta, M.; Yen, C.; Samuel, M.C.; Schechter, R.; Guevara, H.; Uyeki, T.M. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin. Infect. Dis. 2012, 55, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qian, Y.H.; Bao, C.J.; Guo, X.L.; Cui, L.B.; Tang, F.Y.; Ji, H.; Huang, Y.; Cai, P.Q.; Lu, B.; et al. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: Epidemiological investigation. BMJ 2013, 347, f4752. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, Y.; Yu, Z.; Horby, P.W.; Wang, F.; Hu, J.; Yang, X.; Mao, H.; Qin, S.; Chai, C.; et al. A family cluster of three confirmed cases infected with avian influenza A (H7N9) virus in Zhejiang Province of China. BMC Infect. Dis. 2014, 14, 698. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.N.; Yao, H.P.; Liang, W.F.; Wu, X.X.; Wu, H.B.; Wu, N.P.; Yang, S.G.; Zhang, Q.; Su, K.K.; Guo, J.; et al. Viral genome and antiviral drug sensitivity analysis of two patients from a family cluster caused by the influenza A(H7N9) virus in Zhejiang, China, 2013. Int. J. Infect. Dis. 2014, 29, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.F.; Ma, M.J.; Zhan, B.D.; Lai, S.M.; Hu, Y.; Yang, X.X.; Li, J.; Cao, G.P.; Zhou, J.J.; Zhang, J.M.; et al. Nosocomial transmission of avian influenza A (H7N9) virus in China: epidemiological investigation. BMJ 2015, 351, h5765. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | HPAI | L PAI | p3 | p4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H5N1 | H5N6 | H7N9 | H9N2 | |||||||||
| Fatalities (N = 293) | Survivors (N = 151) | p1 | Fatalities (N = 11) | Survivors (N = 5) | Fatalities (N = 265) | Survivors (N = 391) | p2 | Fatalities (N = 0) | Survivors (N = 18) | |||
| Epidemical characteristics | ||||||||||||
| CFR [% (No.)] | 66.0 (293/444) | - | 68.75 (11/16) | 40.4 (265/656) | - | 0.0 (0/18) | <0.001 | - | ||||
| Percentage of countries/provinces reporting fatalities [% (No.)] | 87.5 (14/16) | - | 71.43 (5/7) | 95.0 (19/21) | - | 0.0 (0/4) | 0.587 | - | ||||
| Reported onset date of the first fatality | 2003/11/25 | - | 2014/4/23 | 2013/3/3 | - | 1998 | - | - | ||||
| Reported date of last fatality | 2015/1/12 | - | 2016/11/20 | 2015/5/28 | - | - | - | - | ||||
| Peak season | January | February | - | January | December | January | January | - | - | December | - | - |
| Exposure history [% (No.)] | ||||||||||||
| Any exposure to poultry (Total) | 91.25 (73/80) | 95.4 (103/108) | 0.366 | 100 (11/11) | 100 (5/5) | 49.3 (101/205) | 55.1 (140/254) | 0.223 | - | 77.8 (7/9) | <0.001 | <0.001 |
| Males | 93.0 (40/43) | 92.6 (38/41) | 1.0000 | - | - | 37.2 (45/121) | 47.3 (53/112) | 0.1175 | - | - | - | |
| Females | 89.2 (33/37) | 97.0 (65/67) | 0.1828 | - | - | 44.8 (22/49) | 50.0 (26/52) | 0.6078 | - | - | - | |
| Exposure to sick or dead poultry | 37.5 (30/80) | 25.0 (27/108) | 0.196 | 27.3 (3/11) | 0.0 (0/5) | 5.9 (12/205) | 3.9 (10/254) | 0.379 | - | 11.1 (1/9) | <0.001 | <0.001 |
| Backyard poultry | 25.0 (20/80) | 17.6 (19/108) | 0.275 | 0.0 (0/11) | 0.0 (0/5) | 6.8 (14/205) | 9.1 (23/254) | 0.491 | - | 0.0 (0/9) | <0.001 | 0.030 |
| Visited LBM | 7.5 (6/80) | 8.3 (9/108) | 1.000 | 63.64 (7/11) | 60 (3/5) | 62.9 (129/205) | 50.8 (129/254) | 0.011 | - | 44.4 (4/9) | <0.001 | <0.001 |
| Human case contact | 1.25 (1/80) | 0.9 (1/108) | 0.486 | 0.0 (0/11) | 0.0 (0/5) | 3.9 (8/205) | 7.1 (18/254) | 0.160 | - | 0.0 (0/9) | 1.000 | 0.074 |
| Unknown | 1.25 (1/80) | 5.6 (6/108) | - | 9.09 (1/11) | 40 (2/5) | 10.7 (22/205) | 7.9 (20/254) | - | - | 0.0 (0/9) | - | - |
| Comorbidity [% (No.)] | 18.8 (15/80) | 8.33 (9/108) | 0.046 | 36.36 (4/11) | 0.40 (2/5) | 58.6 (156/266) | 34.8 (135/388) | <0.001 | - | 22.2 (2/9) | <0.001 | <0.001 |
| Gender [Male% (No.)] | 43 (126/293) | 42.3 (41/97) | 1.000 | 45.45 (5/11) | 40.00(2/5) | 70.1 (183/261) | 68.8 (267/388) | 0.795 | - | 33.3 (5/15) | <0.001 | <0.001 |
| Median age (Range, Years) | 22.5 (1–75) | 17 (8 months–75 years) | 0.018 | 39 (25–50) | 35 (5.5–65) | 61 (13–91) | 49 (8 months–88 years) | <0.001 | - | 13 (9 months–86 years) | <0.001 | <0.001 |
| Age group [No. (%),(Years)] | ||||||||||||
| 0–9 | 63 (22) | 42 (43) | <0.001 | 0 (0) | 1 (20) | 0 (0) | 38 (10) | <0.001 | - | 11 (79) | <0.001 | <0.001 |
| 10–19 | 65 (22) | 14 (14) | 0 (0) | 1 (20) | 2 (1) | 7 (2) | - | 1 (7) | ||||
| 20–29 | 76 (26) | 10 (10) | 2 (18.18) | 0 (0) | 10 (4) | 20 (5) | - | - | ||||
| 30–39 | 62 (21) | 15 (15) | 2 (18.18) | 1 (20) | 21 (8) | 54 (14) | - | - | ||||
| 40–49 | 17 (6) | 13 (13) | 6 (54.55) | 0 (0) | 21 (8) | 48 (12) | - | 1 (7) | ||||
| 50–59 | 5 (2) | 1 (1) | 1 (9.09) | 1 (20) | 60 (23) | 92 (24) | - | - | ||||
| Over 60 | 5 (2) | 2 (2) | 0 (0) | 1 (20) | 151 (57) | 128 (33) | - | 1 (7) | ||||
| Median number of days | ||||||||||||
| Days from onset to hospitalization | 5.5 (0–20) | 5 (0–31) | 0.023 | 4 (0–7) | 4.5 (3–6) | 5 (0–31) | 5 (0–28) | 0.761 | - | 2 (1–5) | 0.954 | 0.071 |
| Days from onset to confirmation of infection | 13 (6–29) | 6 (2–17) | <0.001 | 13 (5–20) | 15 (10–20) | 10 (1–51) | 8 (1–28) | 0.011 | - | 17 (2–43) | 0.027 | 0.020 |
| Days from onset to antiviral treatment | 6 (0–14) | 5 (0–31) | 0.202 | 9 (1–14) | 7 (0–12) | 7 (0–23) | 6 (0–19) | 0.089 | - | - | <0.001 | 0.020 |
| Days from onset to outcome | 10 (2–27) | 13 (3–33) | 0.019 | 8 (4–10) | 58 | 23 (3–111) | 31 (4–187) | <0.001 | - | - | <0.001 | <0.001 |
| Hospitalization days | 4 (0–26) | 11 (6–27) | 0.001 | 4 (0–10) | 52 | 18 (0–103) | 25 (1–179) | 0.001 | - | - | <0.001 | 0.044 |
| Groups | HPAI (H5N1) | L PAI (H7N9) | p3 | ||||
|---|---|---|---|---|---|---|---|
| Fatalities (n = 97) | Survivors (n = 132) | p1 | Fatalities (n = 1) | Survivors (n = 42) | p2 | ||
| Percentage of total deaths (%) | 33.1 (97/293) | - | - | 0.4 (1/265) | - | - | 0.030 |
| CFR (%) | 42.5 (97/228) | - | - | 2.4 (1/41) | - | - | <0.001 |
| Male percentage (%) | 46.4 (45/97) | 51.9 (68/131) | 0.410 | 100 (1/1) | 45.2 (19/42) | - | 0.452 |
| Median age (Range, (Years)) | 6.0 (0.9–15) | 4.0 (0.7–15) | <0.001 | 13 | 5.0 (0.75–15) | - | 0.153 |
| Median number of days | |||||||
| Days from onset to hospitalization | 6 (2–13) | 6 (0–25) | 0.963 | 7 | 2.0 (0–8) | - | 0.008 |
| Days from onset to confirmation of infection | 10 (3–15) | 3 (3–14) | 0.034 | 13 | 6.5 (1–67) | - | 0.025 |
| Days from onset to antiviral treatment | 7 (0–14) | 4 (0–25) | 0.044 | 13 | 2.5 (0–13) | - | 0.045 |
| Days from onset to outcome | 13 (3–65) | 10 (6–20) | 0.441 | 17 | 10 (5–15) | - | 0.905 |
| Hospitalization days | 7 (1–61) | 8 (6–18) | 0.596 | 10 | 7 (1–14) | - | 0.271 |
| Characteristics | H5N1 Cluster Fatalities | H7N9 Cluster Fatalities | ||||
|---|---|---|---|---|---|---|
| Index Cases (n = 43) | Secondary Cases (n = 42) | p1 | Index Cases (n = 9) | Secondary Cases (n = 6) | p2 | |
| Percentage of total fatalities (%) | 14.7% (43/293) | 14.3% (42/293) | 0.907 | 3.4% (9/265) | 2.3% (6/265) | 0.432 |
| CFR in clustered cases (%) | 100% (43/43) | 43.3% (42/97) | <0.001 | 37.5% (9/24) | 20.7% (6/29) | 0.176 |
| Median age (range) | 21 (5–69) | 19 (0.75–39) | 0.435 | 56 (37–77) | 54 (21–87) | 0.872 |
| Age group (Years) | ||||||
| 0–9 | 16.3% (7/43) | 16.7% (7/42) | 0.632 | 0.0% (0/9) | 0.0% (0/6) | 0.657 |
| 10–19 | 44.2% (19/43) | 31.0% (13/42) | 0.0% (0/9) | 0.0% (0/6) | ||
| 20–29 | 14.0% (14/43) | 35.7% (15/42) | 0.0% (0/9) | 16.7% (1/9) | ||
| 30–39 | 16.3% (7/43) | 16.7%(7/42) | 11.1% (1/9) | 16.7% (1/9) | ||
| 40–49 | 4.7% (2/43) | 0.0% (0/42) | 11.1% (1/9) | 0.0% (0/6) | ||
| 50–59 | 2.3% (1/43) | 0.0% (0/42) | 44.4% (4/9) | 33.3% (2/9) | ||
| Over 60 | 2.3% (1/43) | 0.0% (0/42) | 33.3% (2/9) | 33.3% (2/9) | ||
| Gender | ||||||
| Female | 65.1% (28/43) | 52.4% (22/42) | 0.233 | 22.2% (2/9) | 33.3% (2/6) | 0.634 |
| Male | 34.9% (15/43) | 47.6% (20/42) | 0.233 | 77.8% (7/9) | 66.7% (4/6) | 0.634 |
| Comorbidities | 9.3% (4/43) | 0.0% (0/42) | 0.043 | 66.7% (6/9) | 50% (3/6) | 0.622 |
| Exposure history | ||||||
| Any exposure to poultry | 67.4% (29/43) | 45.2% (19/42) | 0.039 | 100% (9/9) | 50% (3/6) | 0.018 |
| Common exposure or human case-contact | 0.0% (0/43) | 28.6% (12/42) | <0.001 | 11.1% (1/9) | 100% (6/6) | 0.001 |
| Median number of days | ||||||
| Days from onset to hospitalization | 5 (1–8) | 5 (2–10) | 0.613 | 5 (2–10) | 3 (0–7) | 0.305 |
| Days from onset to confirmation of infection | 11 (7–18) | 12 (6–14) | 0.089 | 10 (6–15) | 9 (6–13) | 0.956 |
| Days from onset to antiviral treatment | 5 (0–10) | 6 (6–12) | 0.057 | 7 (3–12) | 7 (3–12) | 0.781 |
| Days from onset to death | 8 (2–22) | 9(3–14) | 0.450 | 20 (7–57) | 44 (13–85) | 0.085 |
| Hospitalization days | 3.5 (0–16) | 4(1–10) | 0.406 | 16 (1–54) | 40 (10–83) | 0.125 |
| Variable | H5N1 Cases (N = 390) | H7N9 Cases (N = 323) | ||||
|---|---|---|---|---|---|---|
| Value | Odds Ratio (95% CI) | p Value | Value | Odds Ratio (95% CI) | p Value | |
| Male sex, no. (%) | 167/390 (42.82) | 0.85 (0.06–5.95) | 0.465 | 234/323 (72.4) | 0.61 (0.36–1.04) | 0.07 |
| Mean (SD) age, years | 22 (8.5) | 2.58 (0.97–3.68) | <0.001 | 52.08 (20.69) | 1.03 (1.02–1.05) | <0.01 |
| Concurrent health condition, no. (%) | 24/188 (12.77) | 4.15 (2.23–12.49) | <0.001 | 190/323 (58.8) | 1.18 (0.71–1.97) | 0.52 |
| Exposure to poultry history, no. (%) | 176/188 (93.62) | 1.85 (0.98–7.25) | 0.044 | 189/323 (58.5) | 0.73 (0.45–1.18) | 0.20 |
| Median time-to-diagnosis, mo (IQR) | 10 (1–14) | 3.55 (2.14–5.78) | 0.025 | 8 (5) | 1.01 (0.96–1.06) | 0.71 |
| Median time-to- to antiviral drug treatment , mo (IQR) | 5 (1–14) | 1.00 (0.89–1.14) | 0.357 | 6 (4) | 1.00 (0.91–1.09) | 0.93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-Q.; Zhang, Y.; Zhao, N.; Yu, Z.; Pan, H.; Chan, T.-C.; Zhang, Z.-R.; Liu, S.-L. Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses. Int. J. Environ. Res. Public Health 2017, 14, 263. https://doi.org/10.3390/ijerph14030263
Wu Z-Q, Zhang Y, Zhao N, Yu Z, Pan H, Chan T-C, Zhang Z-R, Liu S-L. Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses. International Journal of Environmental Research and Public Health. 2017; 14(3):263. https://doi.org/10.3390/ijerph14030263
Chicago/Turabian StyleWu, Zu-Qun, Yi Zhang, Na Zhao, Zhao Yu, Hao Pan, Ta-Chien Chan, Zhi-Ruo Zhang, and She-Lan Liu. 2017. "Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses" International Journal of Environmental Research and Public Health 14, no. 3: 263. https://doi.org/10.3390/ijerph14030263
APA StyleWu, Z.-Q., Zhang, Y., Zhao, N., Yu, Z., Pan, H., Chan, T.-C., Zhang, Z.-R., & Liu, S.-L. (2017). Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses. International Journal of Environmental Research and Public Health, 14(3), 263. https://doi.org/10.3390/ijerph14030263






