Proximity to Industrial Food Animal Production and Asthma Exacerbations in Pennsylvania, 2005–2012
Abstract
:1. Introduction
2. Materials and Methods
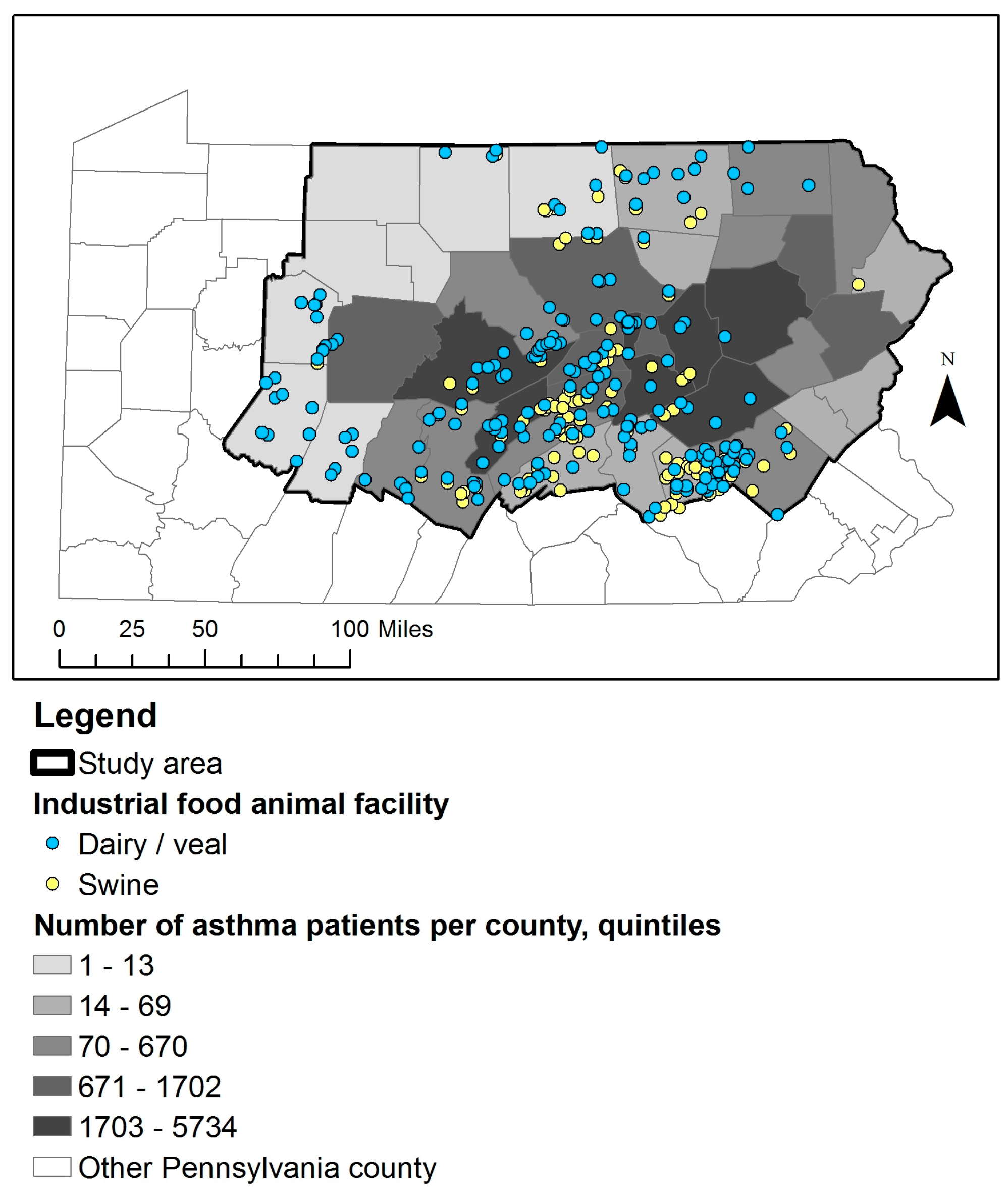
2.1. IFAP Data and Study Population
2.2. Comparison Subjects and Matching
2.3. Statistical Analysis
2.4. Sensitivity Analyses
3. Results
3.1. Description of Study Population
3.2. Association of IFAP and Asthma Exacerbations
3.3. Results of Sensitivity Analyses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casey, J.A.; Kim, B.F.; Larsen, J.; Price, L.B.; Nachman, K.E. Industrial food animal production and community health. Curr. Environ. Health Rep. 2015, 2, 259–271. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute; National Asthma Education Program; Expert Panel on the Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma: Full Report; U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 2007.
- Moorman, J.E.; Akinbami, L.J.; Bailey, C.M.; Johnson, C.A.; King, M.E.; Liu, X.; Zahran, H.S. National surveillance of asthma: United States, 2001–2010. Vital Health Stat. Ser. Anal. Epidemiol. Stud. 2012, 35, 1–58. [Google Scholar]
- Casey, J.A.; Curriero, F.C.; Cosgrove, S.E.; Nachman, K.E.; Schwartz, B.S. High-Density livestock operations, crop field application of manure, and risk of Community-Associated Methicillin-Resistant Staphylococcus Aureus infection in Pennsylvania. JAMA Intern. Med. 2013, 173, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.; Yost, M.; Sampson, P.; Torres, E.; Arias, G.; Breckwich Vasquez, V.; Hartin, K.; Armstrong, J.; Tchong-French, M.; Vedal, S.; et al. Ambient ammonia exposures in an agricultural community and pediatric asthma morbidity. Epidemiology 2015, 26, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.W.; Sears, M.R. Asthma Exacerbations. 1: Epidemiology. Thorax 2006, 61, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Sigurdarson, S.T.; Kline, J.N. School proximity to concentrated animal feeding operations and prevalence of asthma in students. Chest 2006, 129, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.C.; Wing, S.; Marshall, S.W.; Wilcosky, T.C. Asthma symptoms among adolescents who attend public schools that are located near confined swine feeding operations. Pediatrics 2006, 118, e66–e75. [Google Scholar] [CrossRef] [PubMed]
- Pavilonis, B.T.; Sanderson, W.T.; Merchant, J.A. Relative exposure to swine animal feeding operations and childhood asthma prevalence in an agricultural cohort. Environ. Res. 2013, 122, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schinasi, L.; Horton, R.A.; Guidry, V.T.; Wing, S.; Marshall, S.W.; Morland, K.B. Air pollution, lung function, and physical symptoms in communities near concentrated swine feeding operations. Epidemiology 2011, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Borlee, F.; Yzermans, C.J.; van Dijk, C.E.; Heederik, D.; Smit, L.A. Increased respiratory symptoms in COPD patients living in the vicinity of livestock farms. Eur. Respir. J. 2015, 46, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Schulze, A.; Ehrenstein, V.; van Strien, R.T.; Praml, G.; Nowak, D. Environmental exposure to confined animal feeding operations and respiratory health of neighboring residents. Epidemiology 2007, 18, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Rommelt, H.; Ehrenstein, V.; van Strien, R.; Praml, G.; Kuchenhoff, H.; Nowak, D.; Radon, K. Effects on pulmonary health of neighboring residents of concentrated animal feeding operations: Exposure assessed using optimized estimation technique. Arch. Environ. Occup. Health 2011, 66, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Hooiveld, M.; van der Sman-de Beer, F.; Opstal-van Winden, A.W.; Beekhuizen, J.; Wouters, I.M.; Yzermans, C.J.; Heederik, D. Air pollution from livestock farms, and asthma, allergic rhinitis and COPD among neighbouring residents. Occup. Environ. Med. 2014, 71, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, C.E.; Garcia-Aymerich, J.; Carsin, A.E.; Smit, L.A.; Borlee, F.; Heederik, D.J.; Donker, G.A.; Yzermans, C.J.; Zock, J.P. Risk of exacerbations in copd and asthma patients living in the neighbourhood of livestock farms: Observational study using longitudinal data. Int. J. Hyg. Environ. Health 2016, 219, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, C.E.; Smit, L.A.; Hooiveld, M.; Zock, J.; Wouters, I.M.; Heederik, D.J.; Yzermans, C.J. Associations between proximity to livestock farms, primary health care visits and self-reported symptoms. BMC Fam. Pract. 2016, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.G.; Ogburn, E.L.; McCormack, M.; Casey, J.A.; Bandeen-Roche, K.; Mercer, D.G.; Schwartz, B.S. Association between unconventional natural gas development in the marcellus shale and asthma exacerbations. JAMA Intern. Med. 2016, 176, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.A.; Avila, P.C.; Thompson, J.A.; Law, M.; Quraishi, J.A.; Greiman, A.K.; Just, E.M.; Kho, A. A highly specific algorithm for identifying asthma cases and controls for Genome-Wide Association Studies. AMIA Annu. Symp. Proc. 2009, 2009, 497–501. [Google Scholar] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806. [Google Scholar] [CrossRef] [PubMed]
- Nau, C.; Schwartz, B.S.; Bandeen-Roche, K.; Liu, A.; Pollak, J.; Hirsch, A.; Bailey-Davis, L.; Glass, T.A. Community socioeconomic deprivation and obesity trajectories in children using electronic health records. Obesity 2015, 23, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Curriero, F.C.; Glass, T.A.; Stewart, W.F.; Schwartz, B.S. Associations of the burden of coal abandoned mine lands with three dimensions of community context in Pennsylvania. ISRN Public Health 2012. [Google Scholar] [CrossRef]
- Casey, J.A.; Schwartz, B.S.; Stewart, W.F.; Adler, N.E. Using electronic health records for population health research: A review of methods and applications. Annu. Rev. Public Health 2015, 37, 61–81. [Google Scholar] [CrossRef] [PubMed]
| Variable, n (% a), Unless Specified | Hospitalization | Emergency Visit | OCS b | |||
|---|---|---|---|---|---|---|
| Case n = 3552 | Comparison Subject n = 10,640 | Case n = 1445 | Comparison Subject n = 7225 | Case n = 13,137 | Comparison Subject n = 13,044 | |
| Race/ethnicity | ||||||
| White non-Hispanic | 3297 (92.8) | 10,065 (94.6) | 1218 (84.3) | 6720 (93) | 12,126 (92.3) | 11,998 (92) |
| Black non-Hispanic | 107 (3) | 257 (2.4) | 124 (8.6) | 221 (3.1) | 428 (3.3) | 468 (3.6) |
| Hispanic/other/missing | 148 (4.1) | 318 (3.0) | 103 (7.1) | 284 (3.9) | 583 (4.5) | 578 (4.5) |
| p c < 0.01 | p < 0.01 | p = 0.43 | ||||
| Family history of asthma | 404 (11.4) | 999 (9.4) | 265 (18.3) | 871 (12.1) | 1668 (12.7) | 1354 (10.4) |
| p < 0.01 | p < 0.01 | p < 0.01 | ||||
| Smoking status | ||||||
| Never | 1529 (43) | 5567 (52.3) | 656 (45.4) | 4148 (57.4) | 7424 (56.5) | 7975 (61.1) |
| Current | 895 (25.2) | 1913 (18) | 276 (19.1) | 1100 (15.2) | 2219 (16.9) | 1777 (13.6) |
| Former/missing | 1128 (31.8) | 3160 (29.7) | 513 (35.5) | 1977 (27.4) | 3494 (26.6) | 3292 (25.2) |
| p < 0.01 | p < 0.01 | p < 0.01 | ||||
| Medical Assistance | 1094 (30.8) | 2024 (19) | 542 (37.5) | 1991 (27.6) | 3683 (28.0) | 3369 (25.8) |
| p < 0.01 | p < 0.01 | p < 0.01 | ||||
| Body mass index | ||||||
| Normal/underweight | 794 (22.4) | 2873 (27) | 445 (30.8) | 2574 (35.6) | 3887 (29.6) | 4693 (36) |
| Overweight | 786 (22.1) | 2758 (25.9) | 304 (21) | 1708 (23.6) | 3103 (23.6) | 3164 (24.3) |
| Obese | 1972 (55.5) | 5009 (47.1) | 696 (48.2) | 2943 (40.7) | 6147 (46.8) | 5187 (39.8) |
| p < 0.01 | p < 0.01 | p < 0.01 | ||||
| Type 2 diabetes | 558 (15.7) | 1033 (9.7) | 114 (7.9) | 405 (5.6) | 1051 (8.0) | 1010 (7.7) |
| p < 0.01 | p < 0.01 | p = 0.44 | ||||
| Age d (years) | ||||||
| 5–12 | 301 (8.5) | 903 (8.5) | 376 (26) | 1880 (26) | 2975 (22.6) | 2975 (22.8) |
| 13–18 | 222 (6.3) | 666 (6.3) | 156 (10.8) | 780 (10.8) | 1325 (10.1) | 1298 (10) |
| 19–44 | 1347 (37.9) | 4041 (38) | 576 (39.9) | 2880 (39.9) | 4065 (30.9) | 4065 (31.2) |
| 45–61 | 946 (26.6) | 2838 (26.7) | 234 (16.2) | 1170 (16.2) | 3177 (24.2) | 3110 (23.8) |
| 62–74 | 470 (13.2) | 1410 (13.3) | 70 (4.8) | 350 (4.8) | 1146 (8.7) | 1146 (8.8) |
| 75+ | 266 (7.5) | 782 (7.3) | 33 (2.3) | 165 (2.3) | 449 (3.4) | 450 (3.4) |
| Female | 2502 (70.4) | 7506 (70.5) | 868 (60.1) | 4340 (60.1) | 8133 (61.9) | 8040 (61.6) |
| Year | ||||||
| 2005 | 527 (14.8) | 1581 (14.9) | 166 (11.5) | 830 (11.5) | ||
| 2006 | 504 (14.2) | 1512 (14.2) | 147 (10.2) | 735 (10.2) | ||
| 2007 | 436 (12.3) | 1308 (12.3) | 195 (13.5) | 975 (13.5) | ||
| 2008 | 378 (10.6) | 1134 (10.7) | 179 (12.4) | 895 (12.4) | 3356 (25.5) | 3356 (25.7) |
| 2009 | 431 (12.1) | 1293 (12.2) | 206 (14.3) | 1030 (14.3) | 2986 (22.7) | 2986 (22.9) |
| 2010 | 398 (11.2) | 1194 (11.2) | 182 (12.6) | 910 (12.6) | 2405 (18.3) | 2378 (18.2) |
| 2011 | 422 (11.9) | 1266 (11.9) | 174 (12.0) | 870 (12.0) | 2342 (17.8) | 2342 (18.0) |
| 2012 | 456 (12.8) | 1352 (12.7) | 196 (13.6) | 980 (13.6) | 2048 (15.6) | 1982 (15.2) |
| Variable, n (% a), Unless Specified | Hospitalization | Emergency Visit | OCS b | |||
|---|---|---|---|---|---|---|
| Case n = 3552 | Comparison Subject n = 10,640 | Case n = 1445 | Comparison Subject n = 7225 | Case n = 13,137 | Comparison Subject n = 13,044 | |
| IFAP c within 3 miles of home address | 832 (23.4) | 2706 (25.4) | 214 (14.8) | 1849 (25.6) | 3350 (25.5) | 3042 (23.3) |
| p d = 0.02 | p < 0.01 | p < 0.01 | ||||
| Community socioeconomic deprivation, quartiles | ||||||
| 1 | 870 (24.5) | 2839 (26.7) | 289 (20) | 1935 (26.8) | 3424 (26.1) | 3536 (27.1) |
| 2 | 815 (22.9) | 2573 (24.2) | 298 (20.6) | 1728 (23.9) | 3093 (23.5) | 3129 (24.0) |
| 3 | 855 (24.1) | 2694 (25.3) | 345 (23.9) | 1811 (25.1) | 3389 (25.8) | 3203 (24.6) |
| 4 | 1012 (28.5) | 2534 (23.8) | 513 (35.5) | 1751 (24.2) | 3231 (24.6) | 3176 (24.3) |
| p < 0.01 | p < 0.01 | p = 0.06 | ||||
| Distance to nearest major road, miles (mean) | 1.59 | 1.66 | 1.35 | 1.73 | 1.69 | 1.69 |
| p = 0.16 | p < 0.01 | p = 0.91 | ||||
| Distance to nearest minor road, miles (mean) | 0.92 | 1.09 | 0.62 | 1.12 | 1.09 | 1.09 |
| p < 0.01 | p < 0.01 | p = 0.87 | ||||
| Distance to hospital, miles (mean) | 19.4 | 32.4 | 10.3 | 32.9 | 32.5 | 30.8 |
| p < 0.01 | p < 0.01 | p < 0.01 | ||||
| Variable (95% CI a) | Outcome | ||
|---|---|---|---|
| Asthma Hospitalizations b | Asthma Emergency Department Visits b | New Asthma OCS c Orders d | |
| Proximity to industrial food animal production e | 1.29 (1.15–1.46) | 1.12 (0.91–1.37) | 1.11 (1.04–1.19) |
| Race/ethnicity (ref: white non-Hispanic) | |||
| Black non-Hispanic | 1.05 (0.81–1.36) | 1.86 (1.4–2.46) | 0.84 (0.73–0.97) |
| Hispanic | 1.23 (0.99–1.54) | 1.47 (1.11–1.93) | 0.95 (0.84–1.08) |
| Family history of asthma (ref: no) | 1.27 (1.11–1.45) | 1.68 (1.4–2.01) | 1.27 (1.17–1.37) |
| Smoking status (ref: never) | |||
| Current | 1.45 (1.3–1.62) | 1.35 (1.12–1.63) | 1.39 (1.29–1.5) |
| Former | 1.18 (1.08–1.3) | 1.45 (1.25–1.68) | 1.15 (1.08–1.22) |
| Medical Assistance (ref: no) | 1.87 (1.69–2.07) | 1.32 (1.13–1.54) | 1.06 (0.998–1.13) |
| Body mass index (ref: normal/underweight) | |||
| Overweight | 1.07 (0.95–1.21) | 1.07 (0.89–1.28) | 1.24 (1.16–1.33) |
| Obese | 1.36 (1.22–1.51) | 1.3 (1.11–1.52) | 1.53 (1.43–1.62) |
| Type 2 diabetes (ref: no) | 1.63 (1.43–1.85) | 1.29 (0.99–1.69) | 0.93 (0.84–1.03) |
| Community socioeconomic deprivation (ref: quartile 1) | |||
| Quartile 2 | 0.97 (0.83–1.13) | 1.28 (1.01–1.63) | 0.99 (0.91–1.08) |
| Quartile 3 | 0.97 (0.83–1.14) | 1.41 (1.10–1.82) | 1.01 (0.93–1.11) |
| Quartile 4 | 0.94 (0.79–1.12) | 1.41 (1.10–1.82) | 0.98 (0.89–1.08) |
| Distance to nearest major arterial road, z-transformed | |||
| z-transformed | 0.94 (0.84–1.04) | 1.10 (0.93–1.31) | 0.97 (0.91–1.03) |
| squared | 1.07 (1.02–1.12) | 1.04 (0.97–1.12) | 1.02 (0.99–1.05) |
| Distance to nearest minor arterial road | |||
| z-transformed | 1.08 (0.97–1.19) | 1.03 (0.87–1.22) | 0.98 (0.92–1.04) |
| squared | 0.998 (0.95–1.05) | 0.89 (0.79–1.003) | 1.003 (0.98–1.03) |
| Distance to nearest Geisinger hospital | |||
| z-transformed | 0.46 (0.42–0.51) | 0.10 (0.08–0.13) | |
| squared | 1.14 (1.07–1.21) | 1.35 (1.09–1.66) | f |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, S.G.; Casey, J.A.; Bandeen-Roche, K.; Schwartz, B.S. Proximity to Industrial Food Animal Production and Asthma Exacerbations in Pennsylvania, 2005–2012. Int. J. Environ. Res. Public Health 2017, 14, 362. https://doi.org/10.3390/ijerph14040362
Rasmussen SG, Casey JA, Bandeen-Roche K, Schwartz BS. Proximity to Industrial Food Animal Production and Asthma Exacerbations in Pennsylvania, 2005–2012. International Journal of Environmental Research and Public Health. 2017; 14(4):362. https://doi.org/10.3390/ijerph14040362
Chicago/Turabian StyleRasmussen, Sara G., Joan A. Casey, Karen Bandeen-Roche, and Brian S. Schwartz. 2017. "Proximity to Industrial Food Animal Production and Asthma Exacerbations in Pennsylvania, 2005–2012" International Journal of Environmental Research and Public Health 14, no. 4: 362. https://doi.org/10.3390/ijerph14040362






