The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
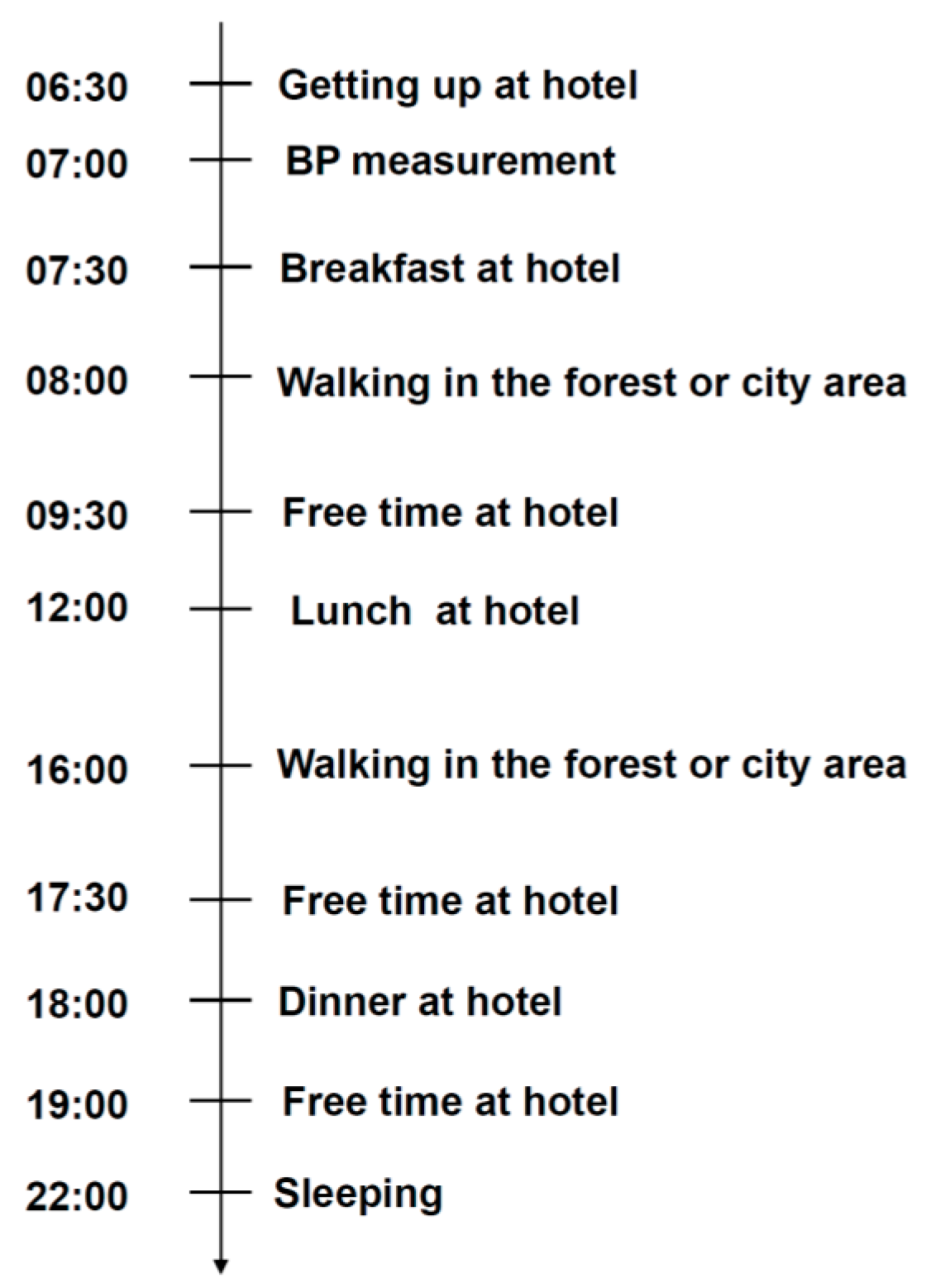
2.2. Study Design
2.3. Bio-Indicators Determination
2.4. Profile of Mood States Evaluation
2.5. Air Quality Assessment
2.6. Data Analysis
3. Results
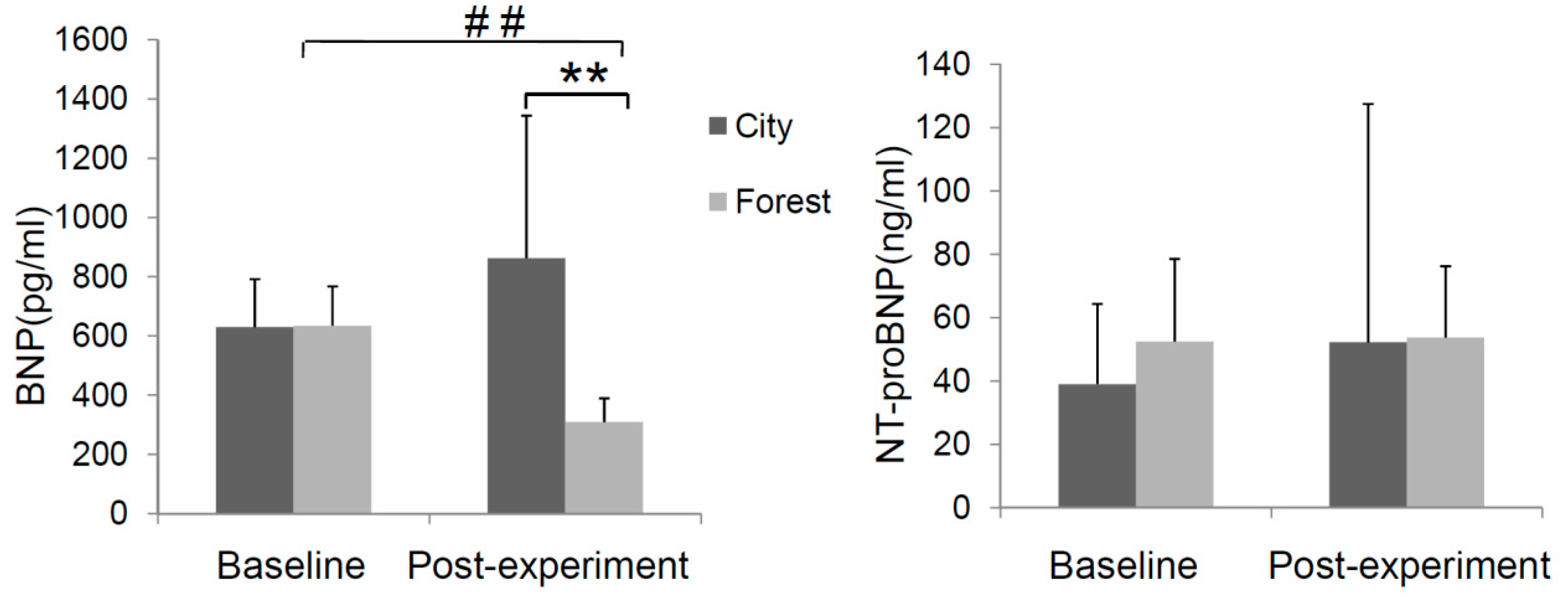
3.1. Effect of Forest Bathing on BNP and NT-ProBNP in Peripheral Blood
3.2. Effect of Forest Bathing on Cardiovascular Disease-Associated Factors
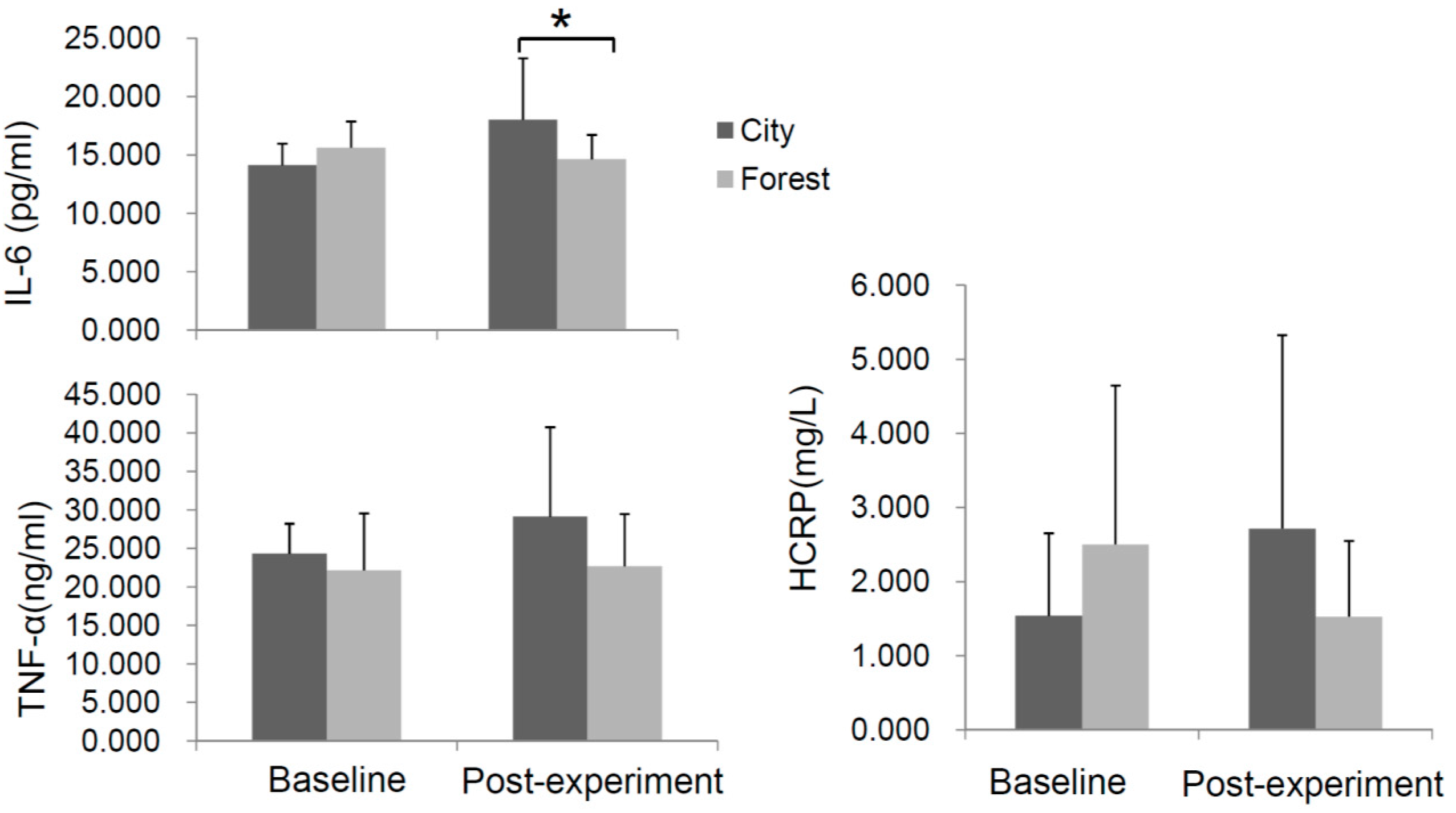
3.3. Effect of Forest Bathing on the Levels of Inflammatory Cytokines in the Serum
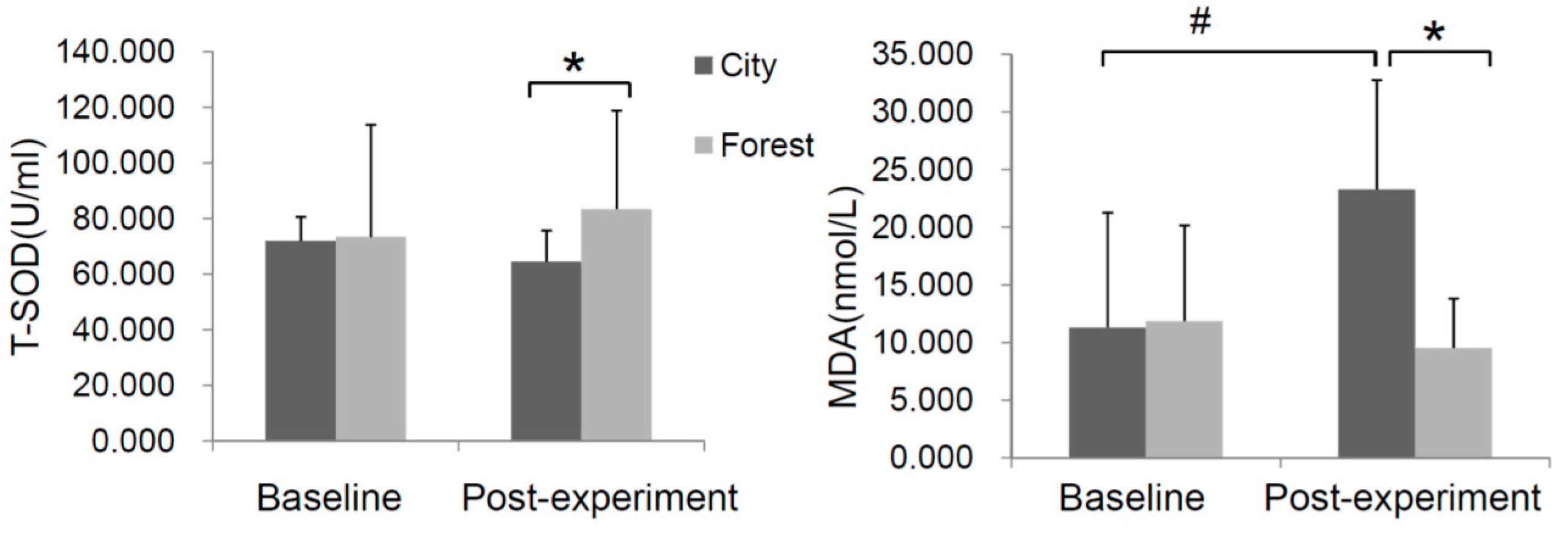
3.4. Effect of Forest Bathing on Oxidative Stress
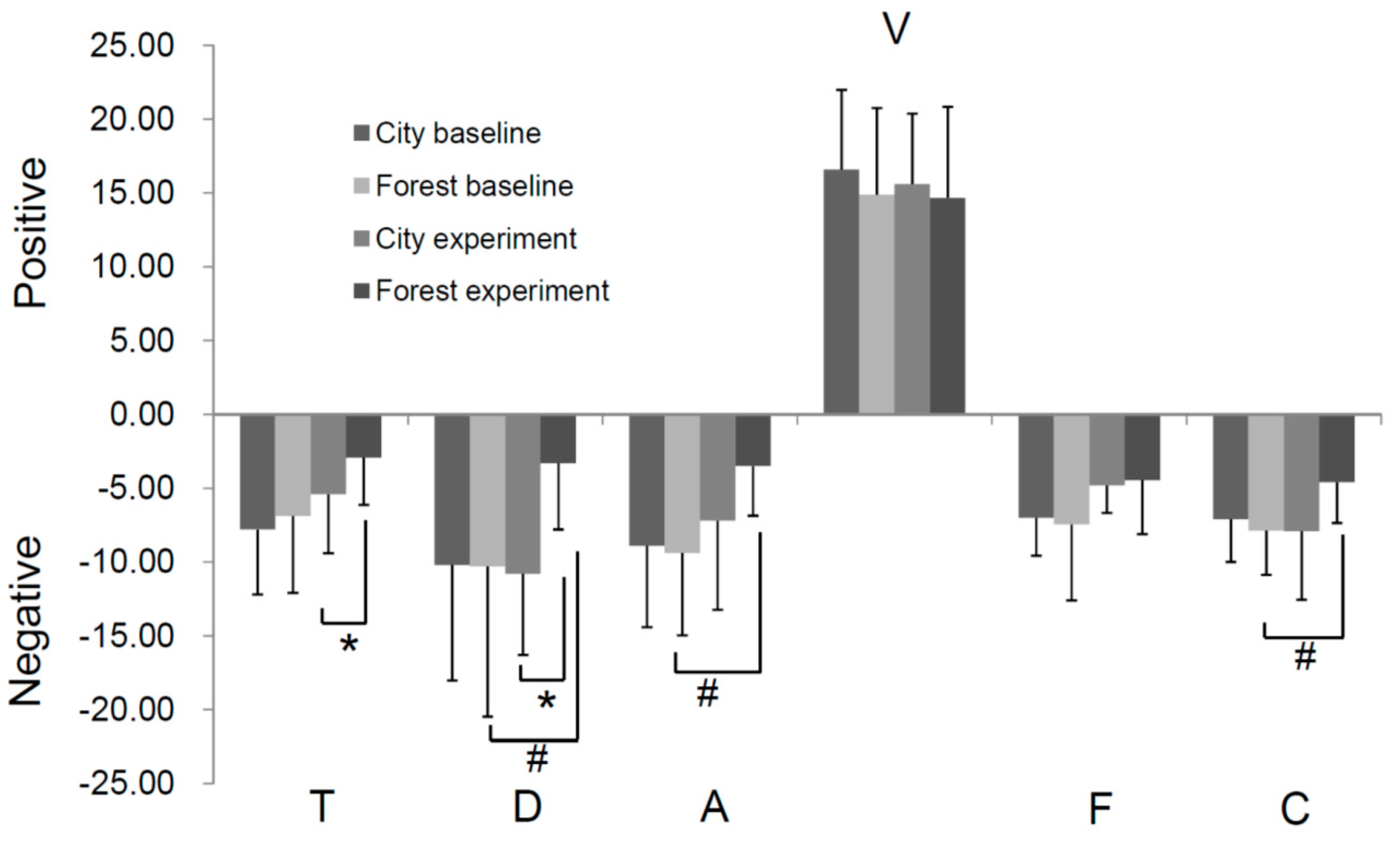
3.5. Effect of Forest Bathing on Mood State of Participants
3.6. Air Quality Assessment
4. Discussion
4.1. Main Founding of This Research
4.2. Relationship of the Beneficial Effect of Forest Bathing and the Environmental Parameters
4.3. Response of Inflammation and Oxidative Stress upon Forest Bathing
4.4. Association of Mood State and Heart Failure
4.5. Limitations of the Experiment
5. Conclusions
Clinical Trial
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001, 104, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics—2016 update: A report from the American heart association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Brunekreef, B.; Goldbohm, S.; Fischer, P.; Van Den Brandt, P.A. Association between mortality and indicators of traffic-related air pollution in the Netherlands: A cohort study. Lancet 2002, 360, 1203–1209. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Xinhua News Agency. The Average Concentration of PM2.5 Was 47 μg/m3 in 2016 in China. Available online: http://www.gov.cn/xinwen/2017-01/05/content_5156570.htm (accessed on 6 March 2017).
- Krewski, D.N. Evaluating the effects of ambient air pollution on life expectancy. N. Engl. J. Med. 2009, 360, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Kioumourtzoglou, M.A.; Hart, J.E.; Okereke, O.I.; Laden, F.; Weisskopf, M.G. The relation between past exposure to fine particulate air pollution and prevalent anxiety: Observational cohort study. BMJ 2015, 350, h1111. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; Molassiotis, A.; Payne, S. What research evidence is there for the use of art therapy in the management of symptoms in adults with cancer? A systematic review. Psychooncology 2011, 20, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.D.; Woo, J.M.; Kim, W.; Lim, S.K.; Chung, E.J. The effect of cognitive behavior therapy-based “forest Therapy” program on blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin. Exp. Hypertens 2012, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lim, S.K.; Chung, E.J.; Woo, J.M. The effect of cognitive behavior therapy based psychotherapy applied in a forest environment on physiological changes and remission of major depressive disorder. Psychiatry Investig. 2009, 6, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.X.; Lan, X.G.; Cao, Y.B.; Chen, Z.M.; He, Z.H.; Lv, Y.D.; Wang, Y.Z.; Hu, X.L.; Wang, G.F.; Yan, J. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China. Biomed. Environ. Sci. 2012, 25, 317–324. [Google Scholar] [PubMed]
- Mao, G.X.; Cao, Y.B.; Lan, X.G.; He, Z.H.; Chen, Z.M.; Wang, Y.Z.; Hu, X.L.; Lv, Y.D.; Wang, G.F.; Yan, J. Therapeutic effect of forest bathing on human hypertension in the elderly. J. Cardiol. 2012, 60, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Park, J.E.; Wort, S.J. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol. Res. 2011, 63, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.B.; Yang, Z.X.; Mao, G.X.; Lyu, Y.D.; Wen, X.L.; Xu, W.H.; Lyu, X.L.; Cao, Y.B.; Wang, G.F. Health effect of forest bathing trip on elderly patients with chronic obstructive pulmonary disease. Biomed. Environ. Sci. 2016, 29, 212–218. [Google Scholar] [PubMed]
- Mao, G.X.; Deng, H.B.; Yuan, L.G.; Li, D.D.; Li, Y.Y.; Wang, Z. Protective role of salidroside against aging in a mouse model induced by d-galactose. Biomed. Environ. Sci. 2010, 23, 161–166. [Google Scholar] [CrossRef]
- Latini, R.; Masson, S.; Anand, I.; Salio, M.; Hester, A.; Judd, D.; Barlera, S.; Maggioni, A.P.; Tognoni, G.; Cohn, J.N.; et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur. Heart J. 2004, 25, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zucker, I.H. AT2 receptor signaling and sympathetic regulation. Curr. Opin. Pharmacol. 2011, 11, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Pollock, V.; Cho, D.W.; Reker, D.; Volavka, J. Profile of mood states: The factors and their physiological correlates. J. Nerv. Ment. Dis. 1979, 167, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Ryushi, T.; Kita, I.; Sakurai, T.; Yasumatsu, M.; Isokawa, M.; Aihara, Y.; Hama, K. The effect of exposure to negative air ions on the recovery of physiological responses after moderate endurance exercise. Int. J. Biometeorol. 1998, 41, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Alexander, D.D.; Bailey, W.H. Air ions and mood outcomes: A reviewand meta-analysis. BMC Psychiatry 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Guinjoan, S.M.; Castro, M.N.; Vigo, D.E.; Weidema, H.; Berbara, C.; Fahrer, R.D.; Grancelli, H.; Nogues, M.; Leiguarda, R.C.; Cardinali, D.P. Depressive symptoms are related to decreased low-frequency heart rate variability in older adults with decompensated heart failure. Neuropsychobiology 2007, 55, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Tager, I.B.; Lurmann, F.; Segal, M.; Quesenberry, C.P., Jr.; Lugg, M.M.; Shan, J.; Van Den Eeden, S.K. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ. Health Perspect. 2002, 110, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.; Baccarelli, A.; Zanobetti, A.; Hoxha, M.; Vokonas, P.S.; Wright, R.O.; Schwartz, J. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology 2014, 25, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Rodosthenous, R.S.; Coull, B.A.; Lu, Q.; Vokonas, P.S.; Schwartz, J.D.; Baccarelli, A.A. Ambient particulate matter and microRNAs in extracellular vesicles: A pilot study of older individuals. Particle Fibre Toxicol. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Wellenius, G.A.; Yeh, G.Y.; Coull, B.A.; Suh, H.H.; Phillips, R.S.; Mittleman, M.A. Effects of ambient air pollution on functional status in patients with chronic congestive heart failure: A repeated-measures study. Environ. Health 2007, 6. [Google Scholar] [CrossRef] [PubMed]
- Hensel, M.; Stuhr, M.; Geppert, D.; Kersten, J.F.; Lorenz, J.; Kerner, T. Relationship between ambient temperature and frequency and severity of cardiovascular emergencies: A prospective observational study based on out-of-hospital care data. Int. J. Cardiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Hwang, S.A.; Kinney, P.L.; Lin, S. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York State. Sci. Total Environ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.Q.; Yang, J.; Ou, Q.Q.; Liu, H.Z.; Lin, G.Z.; Chen, P.Y.; Qian, J.; Guo, Y.M. The impact of relative humidity and atmospheric pressure on mortality in Guangzhou, China. Biomed. Environ. Sci. 2014, 27, 917–925. [Google Scholar] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrvainen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid. Based Complement. Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Van Wagoner, D.R. Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation: An update. J. Cardiovasc. Pharmacol. 2015, 66, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Antman, E.M.; Black, H.R.; Hayes, D.L.; Manson, J.E.; Plutzky, J.; Popma, J.J.; Stevenson, W. The cardiovascular disease continuum validated: Clinical evidence of improved patient outcomes: Part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 2006, 114, 2850–2870. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Fukuda, S.; Nagano, J.; Hamajima, N.; Yamamoto, H.; Iwai, Y.; Nakashima, T.; Ohira, H.; Shirakawa, T. Psychological effects of forest environments on healthy adults: Shinrin-yoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health 2007, 121, 54–63. [Google Scholar] [CrossRef] [PubMed]

| Forest Group (n = 23) | City Group (n = 10) | p Value | |
|---|---|---|---|
| Age (years) | 72.86 ± 5.85 | 70.70 ± 3.68 | 0.341 |
| Gender (M/F) | 12/11 | 7/3 | 0.455 * |
| Hight (cm) | 162.45 ± 8.56 | 163.50 ± 9.01 | 0.755 |
| Weight (kg) | 65.18 ± 7.40 | 65.20 ± 10.90 | 0.996 |
| BMI (kg/m2) | 24.72 ± 2.38 | 24.35 ± 3.45 | 0.722 |
| SBP (mmHg) | 141.4 ± 15.1 | 142.5 ± 15.7 | 0.849 |
| DBP (mmHg) | 80.6 ± 10.5 | 75.9 ± 14.1 | 0.296 |
| HR (bmp) | 76.2 ± 11.5 | 69.0 ± 12.2 | 0.113 |
| New York Heart Association Class | 0.845 * | ||
| I | 1 (4.3%) | 1 (10.0%) | |
| II | 16 (69.6%) | 6 (60.0%) | |
| III | 6 (26.1%) | 3 (30.0%) |
| City Site | Forest Site | p Value | |
|---|---|---|---|
| Negative ion (cm−3) | 397.6 ± 230.8 | 11,575.0 ± 1399.7 | 0.003 * |
| PM2.5 (μg/m3) | 160.1 ± 41.3 | 9.4 ± 4.1 | <0.001 |
| T (°C) | 29.3 ± 4.3 | 25.2 ± 2.5 | 0.040 |
| RH (%) | 51.8 ± 15.2 | 77.3 ± 3.7 | 0.042 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, G.; Cao, Y.; Wang, B.; Wang, S.; Chen, Z.; Wang, J.; Xing, W.; Ren, X.; Lv, X.; Dong, J.; et al. The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure. Int. J. Environ. Res. Public Health 2017, 14, 368. https://doi.org/10.3390/ijerph14040368
Mao G, Cao Y, Wang B, Wang S, Chen Z, Wang J, Xing W, Ren X, Lv X, Dong J, et al. The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure. International Journal of Environmental Research and Public Health. 2017; 14(4):368. https://doi.org/10.3390/ijerph14040368
Chicago/Turabian StyleMao, Genxiang, Yongbao Cao, Bozhong Wang, Sanying Wang, Zhuomei Chen, Jirong Wang, Wenmin Xing, Xiaoxu Ren, Xiaoling Lv, Jianhua Dong, and et al. 2017. "The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure" International Journal of Environmental Research and Public Health 14, no. 4: 368. https://doi.org/10.3390/ijerph14040368
APA StyleMao, G., Cao, Y., Wang, B., Wang, S., Chen, Z., Wang, J., Xing, W., Ren, X., Lv, X., Dong, J., Chen, S., Chen, X., Wang, G., & Yan, J. (2017). The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure. International Journal of Environmental Research and Public Health, 14(4), 368. https://doi.org/10.3390/ijerph14040368




