The Prevalence of Hypertension Accompanied by High Homocysteine and its Risk Factors in a Rural Population: A Cross-Sectional Study from Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Statement
2.3. Data Collection
2.4. Smoking Status
2.5. Diet Score
2.6. Physical Activity
2.7. Category of Blood Pressure
2.8. Category of BMI
2.9. Serum Analysis
2.10. Estimated Glomerular Filtration Rate
2.11. Simple Hypertension, HHcy and Hypertension Accompanied by HHcy
2.12. Statistical Analyses
3. Results
4. Discussion
5. Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ezzati, M.; Lopez, A.D.; Rodgers, A.; Vander Hoorn, S.; Murray, C.J.; Comparative Risk Assessment Collaborating, G. Selected major risk factors and global and regional burden of disease. Lancet 2002, 360, 1347–1360. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart, A.; Obesity Committee of the Council on Nutrition, P.A. Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [PubMed]
- Li, Z.; Guo, X.; Zheng, L.; Yang, H.; Sun, Y. Grim status of hypertension in rural china: Results from northeast china rural cardiovascular health study 2013. J. Am. Soc. Hypertens. 2015, 9, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Report on Cardiovascular Diseases in China 2015. Available online: http://www.nccd.org.cn/UploadFile/201607/20160718113619135135.pdf (access on 2 April 2017).
- Available online: http://www.nhfpc.gov.cn/xcs/s3574/201506/6b4c0f873c174ace9f57f11fd4f6f8d9.shtml (access on 2 April 2017).
- Hu, D.Y.; Xu, X.P. Prevention of stroke relies on valid control “h” type hypertension. Zhonghua. Nei. Ke. Za. Zhi. 2008, 47, 976–977. [Google Scholar] [PubMed]
- Durand, P.; Prost, M.; Loreau, N.; Lussier-Cacan, S.; Blache, D. Impaired homocysteine metabolism and atherothrombotic disease. Lab. Invest. 2001, 81, 645–672. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Adams, R.; Albers, G.; Alberts, M.J.; Benavente, O.; Furie, K.; Goldstein, L.B.; Gorelick, P.; Halperin, J.; Harbaugh, R.; et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association council on stroke: Co-sponsored by the council on cardiovascular radiology and intervention: The american academy of neurology affirms the value of this guideline. Stroke 2006, 37, 577–617. [Google Scholar] [PubMed]
- Waskiewicz, A.; Sygnowska, E.; Broda, G. Homocysteine concentration and the risk of death in the adult polish population. Kardiol. Pol 2012, 70, 897–902. [Google Scholar] [PubMed]
- Casas, J.P.; Bautista, L.E.; Smeeth, L.; Sharma, P.; Hingorani, A.D. Homocysteine and stroke: Evidence on a causal link from mendelian randomisation. Lancet 2005, 365, 224–232. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Trabetti, E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J. Appl. Genet. 2008, 49, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, Y.; Sun, N.; Wang, H.; Zhang, Y.; Wang, J.; Li, J.; Xu, X.; Liang, M.; Nie, J.; et al. Elevated homocysteine concentrations decrease the antihypertensive effect of angiotensin-converting enzyme inhibitors in hypertensive patients. Arterioscler. Thromb. Vasc. Biol. 2016. [Google Scholar] [CrossRef]
- Guo, S.; Pang, H.; Guo, H.; Zhang, M.; He, J.; Yan, Y.; Niu, Q.; Muratbek; Rui, D.; Li, S.; et al. Ethnic differences in the prevalence of high homocysteine levels among low-income rural Kazakh and Uyghur adults in far western china and its implications for preventive public health. Int. J. Environ. Res. Public Health 2015, 12, 5373–5385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qiu, D.X.; Fu, R.L.; Xu, T.F.; Jing, M.J.; Zhang, H.S.; Geng, H.H.; Zheng, L.C.; Wang, P.X. H-type hypertension and C reactive protein in recurrence of ischemic stroke. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, F.; Zheng, Y.; Zeng, Q.; Liu, H. H-type hypertension is an important risk factor of carotid atherosclerotic plaques. Clin. Exp. Hypertens. 2016, 38, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guo, X.; Chen, Y.; Guo, L.; Li, Z.; Yu, S.; Yang, H.; Sun, G.; Sun, Y. Prevalence and metrics distribution of ideal cardiovascular health: A population-based, cross-sectional study in rural china. Heart Lung Circ. 2016, 25, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, Y.; Guo, X.; Dai, D.; Sun, Y. The association of ideal cardiovascular health and atherogenic index of plasma in rural population: A cross-sectional study from northeast china. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, X.; Bai, Y.; Sun, G.; Guan, Y.; Sun, Y.; Roselle, A.M. The association between alcohol consumption and left ventricular ejection fraction: An observational study on a general population. Medicine (Baltimore) 2016, 95, e3763. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Shi, R.; Hou, B.; Wu, L.; Zheng, L.; Ding, L.; Chen, G.; Zhang, S.; Yao, Y. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, P.D.; Saitz, R.; Gogineni, A.; Zhang, J.X.; Stein, M.D. Validation of the screening strategy in the NIAAA “physicians’ guide to helping patients with alcohol problems”. J. Stud. Alcohol 2001, 62, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ge, K. The transition of chinese dietary guidelines and food guide pagoda. Asia Pac. J. Clin. Nutr. 2011, 20, 439–446. [Google Scholar] [PubMed]
- Hu, G.; Tuomilehto, J.; Silventoinen, K.; Barengo, N.; Jousilahti, P. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur. Heart J. 2004, 25, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Circulation 2005, 111, 697–716. [Google Scholar] [PubMed]
- World Health Organization Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar]
- Liu, X.; Wang, Y.; Wang, C.; Shi, C.; Cheng, C.; Chen, J.; Ma, H.; Lv, L.; Li, L.; Lou, T. A new equation to estimate glomerular filtration rate in chinese elderly population. PLoS One 2013, 8, e79675. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Kucuksu, M.; Kaman, D.; Ilhan, N.; Ozbay, Y. The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch. Med. Res. 2008, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Zhu, J.H.; Hao, L.; Yang, Q.H.; Yang, T.P.; Gindler, J.; Maneval, D.R.; Quinlivan, E.P.; Li, Z.; Bailey, L.B.; et al. MTHFR 677c->t genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am. J. Clin. Nutr. 2011, 93, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ma, J.; Zhu, J.; Stampfer, M.J.; Tian, Y.; Willett, W.C.; Li, Z. High prevalence of hyperhomocysteinemia in chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J. Nutr. 2007, 137, 407–413. [Google Scholar] [PubMed]
- Li, J.P.; Huo, Y.; Liu, P. [efficacy and safety of enalapril-folate acid tablets in lowering blood pressure and plasma homocysteine]. Beijing Da Xue. Xue. Bao. 2007, 39, 614–618. [Google Scholar] [PubMed]
- De Bree, A.; van der Put, N.M.; Mennen, L.I.; Verschuren, W.M.; Blom, H.J.; Galan, P.; Bates, C.J.; Herrmann, W.; Ullrich, M.; Dierkes, J.; et al. Prevalences of hyperhomocysteinemia, unfavorable cholesterol profile and hypertension in European populations. Eur. J. Clin. Nutr. 2005, 59, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fakhrzadeh, H.; Ghotbi, S.; Pourebrahim, R.; Nouri, M.; Heshmat, R.; Bandarian, F.; Shafaee, A.; Larijani, B. Total plasma homocysteine, folate, and vitamin B12 status in healthy Iranian adults: The tehran homocysteine survey (2003–2004)/a cross-sectional population based study. BMC Public Health 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Huo, Y. H-type hypertension, stroke and diabetes in china: Opportunities for primary prevention. J. Diabetes 2016, 8, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, S.; Zhang, Y.; Tang, G.; Wang, Y.; Mao, G.; Li, Z.; Xu, X.; Wang, B.; Huo, Y. H-type hypertension and risk of stroke in chinese adults: A prospective, nested case-control study. J. Transl. Int. Med. 2015, 3, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hu, X.; Zhang, Q.; Li, J.; Liu, B.; Wang, J.; Shao, Y.; Zhang, Z.; Liu, C.; Hu, H.; et al. Hyperhomocysteinaemia, low folate concentrations and MTHFR C677T mutation in abdominal aortic aneurysm. Vasa 2014, 43, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in china: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Wang, F.; Liu, L.; Wang, H. China National Survey of Chronic Kidney Disease Working, G. Prevalence, awareness, treatment, and control of hypertension in china: Results from a national survey. Am. J. Hypertens. 2014, 27, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, B.; Wang, W.Z.; Lee, L.M.; Zhang, S.H.; Kong, L.Z. Stroke in china: Epidemiology, prevention, and management strategies. Lancet Neurol. 2007, 6, 456–464. [Google Scholar] [CrossRef]
- Guo, L.; Guo, X.; Chang, Y.; Li, Z.; Yu, S.; Yang, H.; Sun, Y. Modified ideal cardiovascular health status is associated with lower prevalence of stroke in rural northeast china. Int. J. Environ. Res. Public Health 2016, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Luo, J. Etiopathogenesis and pathogenesis of H type hypertension. Adv. Cardiovasc. Dis. 2012, 33, 253–256. [Google Scholar]
- Towfighi, A.; Markovic, D.; Ovbiagele, B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: A nationwide study. J. Neurol. Sci. 2010, 298, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huxley, R.; Li, L.; Anna, V.; Xie, G.; Yao, C.; Woodward, M.; Li, X.; Chalmers, J.; Gao, R.; et al. Prevalence, awareness, treatment, and control of hypertension in china: Data from the china National Nutrition and Health Survey 2002. Circulation 2008, 118, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.; Mishra, P.K.; Tyagi, N.; Tyagi, S.C. Homocysteine to hydrogen sulfide or hypertension. Cell. Biochem. Biophys. 2010, 57, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Jiang, X.; Yang, F.; Li, Z.; Liao, D.; Trial, J.; Magera, M.J.; Durante, W.; Yang, X.; Wang, H. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc. Res. 2006, 69, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.D.; Chen, I.; Yang, F.; Jiang, X.; Jan, M.; Liu, X.; Schafer, A.I.; Durante, W.; Yang, X.; Wang, H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin a gene. Blood 2007, 110, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Jiang, X.; Kruger, W.D.; Pratico, D.; Gupta, S.; Mallilankaraman, K.; Madesh, M.; Schafer, A.I.; Durante, W.; Yang, X.; et al. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood 2011, 118, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fang, P.; Jiang, X.; Nelson, J.; Moore, J.K.; Kruger, W.D.; Berretta, R.M.; Houser, S.R.; Yang, X.; Wang, H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLR/CBS-deficient mice. Circ. Res. 2012, 111, 37–49. [Google Scholar] [CrossRef] [PubMed]
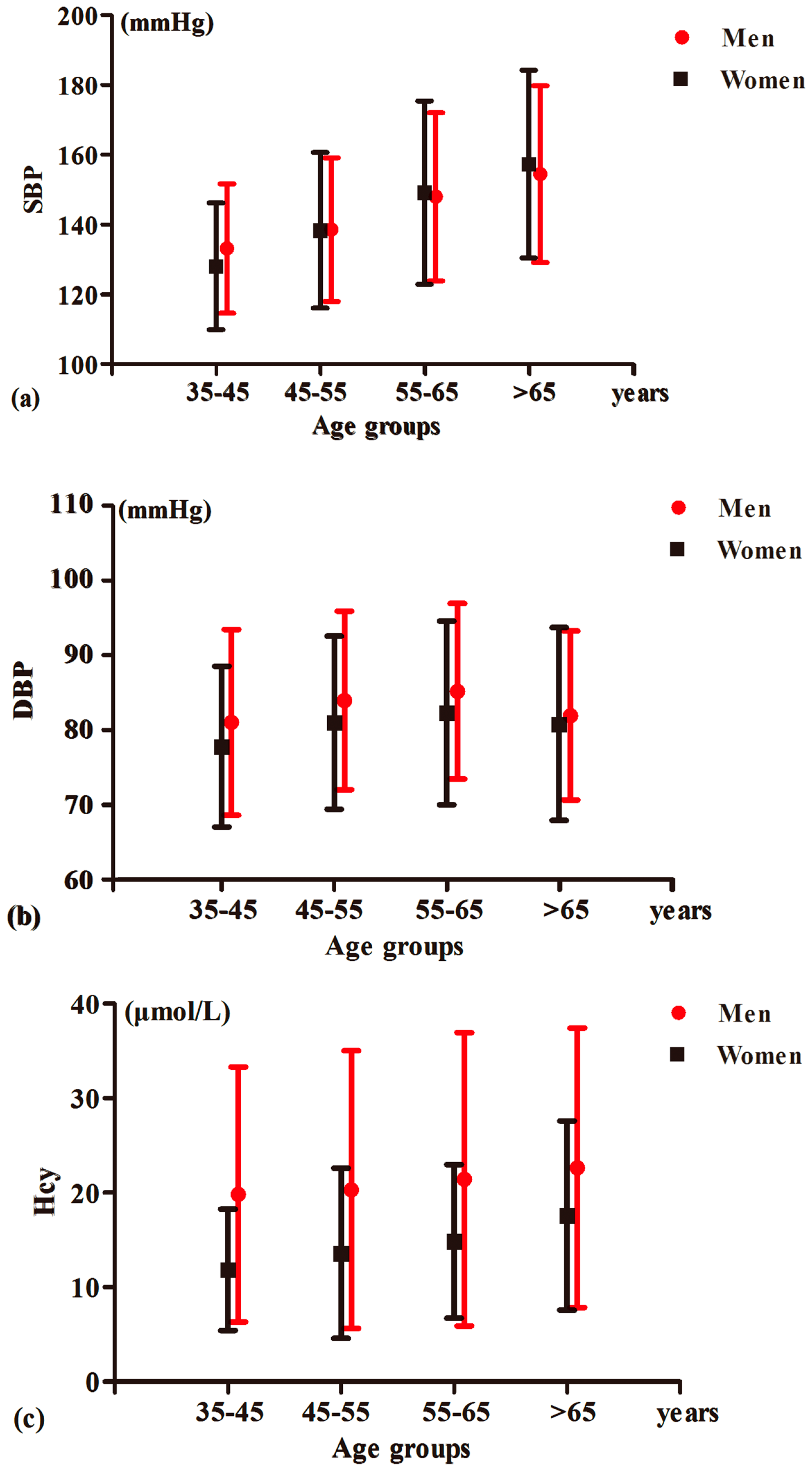
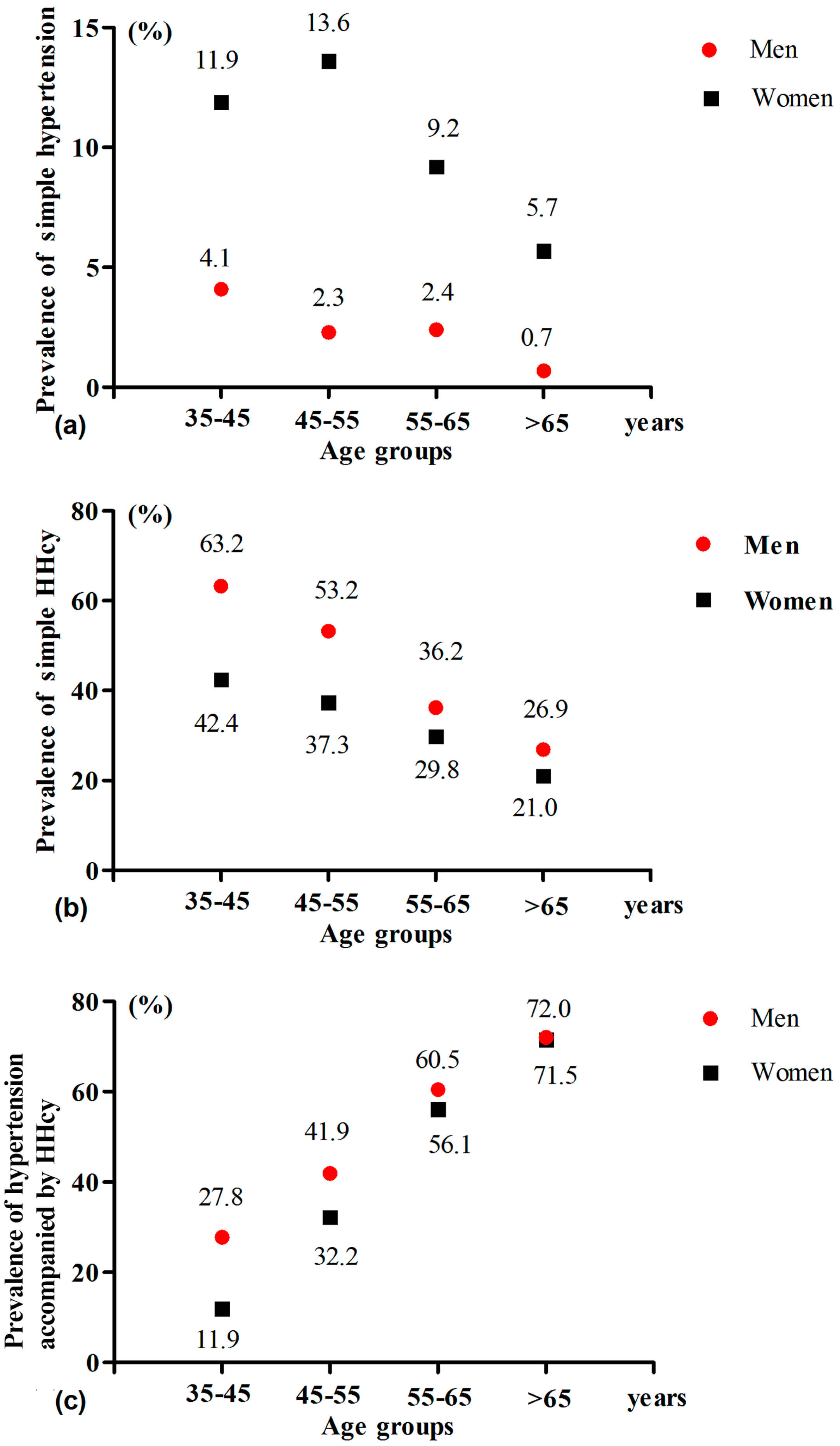
| Variables | Control Group 1 | Simple Hypertension Group | Simple HHcy Group | Hypertension Accompanied by HHcy Group | p Value |
|---|---|---|---|---|---|
| N = 592 | N = 449 | N = 2545 | N = 2943 | ||
| Age (year) | 45.8 ± 7.8 | 51.5 ± 8.8 | 51.8 ± 10.0 | 59.0 ± 10.1 | <0.001 |
| Male (%) | 65 (11.0) | 72 (16.0) | 1352 (53.1) | 1501 (51.0) | <0.001 |
| Spouse (live, %) | 568 (95.9) | 423 (94.2) | 2373 (93.2) | 2549 (86.6) | <0.001 |
| Education (%) | <0.001 | ||||
| Primary school or below | 198 (33.4) | 230 (51.2) | 1151 (45.2) | 1662 (56.5) | |
| Middle school | 341 (57.6) | 180 (40.1) | 1156 (45.4) | 1030 (35.0) | |
| High school or above | 53 (9.0) | 39 (8.7) | 238 (9.4) | 251 (8.5) | |
| Family income (%) | <0.001 | ||||
| ≤5000 (CNY/year) | 46 (7.8) | 67 (14.9) | 381 (15.0) | 678 (23.0) | |
| 5000–20,000 (CNY/year) | 340 (57.4) | 255 (56.8) | 1414 (55.6) | 1690 (57.4) | |
| >20,000 (CNY/year) | 206 (34.8) | 127 (28.3) | 750 (29.5) | 575 (19.5) | |
| Race | 0.022 | ||||
| Han | 547 (92.4) | 413(92.0) | 2415 (94.9) | 2772 (94.2) | |
| Others a | 45 (7.6) | 36 (8.0) | 130 (5.1) | 171 (5.8) | |
| Current smoking (%) | 106 (17.9) | 108 (24.1) | 1088 (42.8) | 1135 (38.6) | <0.01 |
| Current drinking (%) | 50 (8.4) | 56 (12.5) | 615 (24.2) | 730 (24.8) | <0.01 |
| Diet score | 2.2 ± 1.1 | 2.2 ± 1.2 | 2.3 ± 1.2 | 2.2 ± 1.2 | 0.059 |
| eGFR (mL/min/1.73 m2) | 104.3 ± 12.0 | 103.2 ± 13.2 | 94.6 ± 14.5 | 90.2 ± 15.9 | <0.001 |
| SBP (mmHg) | 123.1 ± 10.4 | 157.2 ± 17.5 | 123.7 ± 10.0 | 161.0 ± 20.9 | <0.001 |
| DBP (mmHg) | 74.0 ± 7.5 | 87.5 ± 9.9 | 74.6 ± 7.6 | 88.4 ± 11.6 | <0.001 |
| BMI (kg/m2) | 24.6 ± 4.0 | 25.9 ± 3.7 | 23.8 ± 3.5 | 25.5 ± 3.8 | <0.001 |
| WC (cm) | 80.2 ± 9.4 | 81.8 ± 10.7 | 82.3 ± 8.9 | 83.3 ± 9.4 | <0.001 |
| FPG (mmol/L) | 5.58 ± 1.71 | 6.27 ± 2.36 | 5.61 ± 1.27 | 6.10 ± 1.92 | <0.001 |
| TC (mmol/L) | 4.85 ± 1.09 | 5.13 ± 1.12 | 4.90 ± 0.95 | 5.28 ± 1.05 | <0.001 |
| TG (mmol/L) | 1.47 ± 2.19 | 1.85 ± 2.44 | 1.46 ± 1.21 | 1.84 ± 1.59 | <0.001 |
| LDL (mmol/L) | 2.66 ± 0.69 | 2.89 ± 0.81 | 2.67 ± 0.69 | 2.99 ± 0.82 | <0.001 |
| HDL (mmol/L) | 1.47 ± 0.38 | 1.50 ± 0.41 | 1.41 ± 0.37 | 1.44 ± 0.43 | <0.001 |
| Cr (mmol/L) | 59.77 ± 10.07 | 57.37 ± 11.76 | 72.27 ± 12.64 | 72.30 ± 29.01 | <0.001 |
| BuN(mmol/L) | 5.19 ± 2.07 | 5.16 ± 1.42 | 5.68 ± 2.10 | 5.82± 2.08 | <0.001 |
| UA(μmol/L) | 242.3 ± 65.3 | 249.9 ± 73.5 | 299.5 ± 84.7 | 306.8 ± 89.5 | <0.001 |
| Hcy (μmol/L) | 8.33 ± 1.46 | 8.28 ± 1.55 | 17.83 ± 10.86 | 20.02 ± 14.00 | <0.001 |
| Variables | Control Group 1 | Simple Hypertension Group | Simple HHcy Group | Hypertension Accompanied by HHcy Group | p Value |
|---|---|---|---|---|---|
| N = 592 | N = 449 | N = 2545 | N = 2943 | ||
| Current smoking | <0.001 | ||||
| Ideal | 486 (82.1) | 334 (74.7) | 1432 (56.3) | 1778 (60.4) | |
| Intermediate | 0 (0) | 7 (1.6) | 25 (1.0) | 30 (1.0) | |
| Poor | 106 (17.9) | 108 (24.1) | 1088 (42.7) | 1135 (38.6) | |
| BMI | <0.001 | ||||
| Ideal | 214 (36.1) | 87 (19.4) | 1093 (42.9) | 764 (26.0) | |
| Intermediate | 263 (44.4) | 224 (49.9) | 1092 (42.9) | 1385 (47.0) | |
| Poor | 115 (19.4) | 138 (30.7) | 360 (14.2) | 794 (27.0) | |
| Physical activity | <0.001 | ||||
| Ideal | 327 (55.2) | 246 (54.8) | 1496 (58.8) | 1243 (42.2) | |
| Intermediate | 109 (18.4) | 96 (21.4) | 504 (19.8) | 569 (19.3) | |
| Poor | 156 (26.4) | 107 (23.8) | 545 (21.4) | 1131 (38.4) | |
| Diet score | 0.205 | ||||
| Ideal | 54 (9.1) | 54 (12.0) | 297 (11.7) | 318 (10.8) | |
| Intermediate | 374 (63.2) | 266 (59.2) | 1570 (61.7) | 1762 (59.9) | |
| Poor | 164 (27.7) | 129 (28.8) | 678 (26.6) | 863 (29.3) | |
| TC | <0.001 | ||||
| Ideal | 403 (68.1) | 257 (57.2) | 1650 (64.8) | 1477 (50.2) | |
| Intermediate | 136 (23.0) | 128 (28.5) | 655 (25.7) | 961 (32.7) | |
| Poor | 53 (8.9) | 64 (14.3) | 240 (9.4) | 505 (17.2) | |
| FPG | <0.001 | ||||
| Ideal | 419 (70.8) | 210 (46.8) | 1584 (62.2) | 1366 (46.4) | |
| Intermediate | 144 (24.3) | 172 (38.3) | 824 (32.4) | 1192 (40.5) | |
| Poor | 29 (4.9) | 67 (14.9) | 137 (5.4) | 385 (13.1) |
| Variables | OR (95% CI) | |
|---|---|---|
| Men | Women | |
| TG (per 1 mmol/L) | 1.12 (1.06, 1.19) | 1.09 (1.02, 1.16) |
| LDL(per 1 mmol/L) | 1.60 (1.34, 1.90) | 1.69 (1.43, 1.99) |
| BMI | ||
| Ideal | 1 (Reference) | 1 (Reference) |
| Intermediate | 2.08 (1.71, 2.52) | 1.85 (1.52, 2.25) |
| Poor | 3.41 (2.63, 4.41) | 3.12 (2.45, 3.96) |
| TC | ||
| Ideal | 1 (Reference) | 1 (Reference) |
| Intermediate | 1.27 (0.92, 1.77) | 1.79 (1.35, 2.39) |
| Poor | 1.88 (1.24, 2.85) | 2.28 (1.57, 3.31) |
| FPG | ||
| Ideal | 1 (Reference) | 1 (Reference) |
| Intermediate | 1.29 (1.09, 1.53) | 1.11 (0.94, 1.32) |
| Poor | 1.96 (1.45, 2.65) | 1.39 (1.06, 1.82) |
| Variables | OR (95% CI) | |||
|---|---|---|---|---|
| 35–45 years | 45–55 years | 55–65 years | >65 years | |
| TG (per 1 mmol/L) | 1.14 (1.04, 1.26) | 1.15 (1.06, 1.25) | 1.13 (1.04, 1.22) | 1.09 (0.99, 1.21) |
| LDL (per 1 mmol/L) | 2.03 (1.56, 2.64) | 1.66 (1.36, 2.02) | 1.84 (1.48, 2.29) | 1.87 (1.39, 2.50) |
| BMI | ||||
| Ideal | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 1.47 (1.10, 1.97) | 1.71 (1.35, 2.16) | 2.15 (1.68, 2.75) | 1.85 (1.32, 2.60) |
| Poor | 2.47 (1.71, 3.55) | 2.31 (1.70, 3.13) | 3.44 (2.50, 4.73) | 3.16 (2.08, 4.80) |
| Physical activity | ||||
| Ideal | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 0.98 (0.72, 1.34) | 1.17 (0.90, 1.51) | 1.06 (0.81, 1.38) | 0.81 (0.56, 1.19) |
| Poor | 1.61 (1.20, 2.94) | 1.60 (1.27, 2.02) | 1.42 (1.10, 1.82) | 1.58 (1.13, 2.21) |
| Diet score | ||||
| Ideal | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 1.61 (1.10, 2.36) | 1.02 (0.74, 1.42) | 0.78 (0.56, 1.08) | 0.81 (0.51, 1.27) |
| Poor | 1.97 (1.28, 3.03) | 1.06 (0.74, 1.52) | 0.77 (0.53, 1.11) | 0.83 (0.50, 1.38) |
| TC | ||||
| Ideal | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 1.58 (0.99, 2.51) | 1.77 (1.23, 2.56) | 1.56 (1.06, 2.31) | 1.96 (1.14, 3.37) |
| Poor | 2.56 (1.43, 4.61) | 2.04 (1.27, 3.27) | 2.28 (1.37, 3.80) | 2.56 (1.28, 5.12) |
| FPG | ||||
| Ideal | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Intermediate | 1.44 (1.12, 1.86) | 1.37 (1.11, 1.70) | 1.04 (0.84, 1.30) | 1.38 (1.03, 1.86) |
| Poor | 1.88 (1.20, 2.94) | 1.97 (1.42, 2.75) | 1.76 (1.22, 2.53) | 1.51 (0.88, 2.58) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Li, Y.; Guo, X.; Chen, Y.; Dai, D.; Sun, Y. The Prevalence of Hypertension Accompanied by High Homocysteine and its Risk Factors in a Rural Population: A Cross-Sectional Study from Northeast China. Int. J. Environ. Res. Public Health 2017, 14, 376. https://doi.org/10.3390/ijerph14040376
Chang Y, Li Y, Guo X, Chen Y, Dai D, Sun Y. The Prevalence of Hypertension Accompanied by High Homocysteine and its Risk Factors in a Rural Population: A Cross-Sectional Study from Northeast China. International Journal of Environmental Research and Public Health. 2017; 14(4):376. https://doi.org/10.3390/ijerph14040376
Chicago/Turabian StyleChang, Ye, Yuan Li, Xiaofan Guo, Yintao Chen, Dongxue Dai, and Yingxian Sun. 2017. "The Prevalence of Hypertension Accompanied by High Homocysteine and its Risk Factors in a Rural Population: A Cross-Sectional Study from Northeast China" International Journal of Environmental Research and Public Health 14, no. 4: 376. https://doi.org/10.3390/ijerph14040376
APA StyleChang, Y., Li, Y., Guo, X., Chen, Y., Dai, D., & Sun, Y. (2017). The Prevalence of Hypertension Accompanied by High Homocysteine and its Risk Factors in a Rural Population: A Cross-Sectional Study from Northeast China. International Journal of Environmental Research and Public Health, 14(4), 376. https://doi.org/10.3390/ijerph14040376




