A Comparative Health Risk Assessment of Electronic Cigarettes and Conventional Cigarettes
Abstract
:1. Introduction
2. Methods
2.1. Methodology
2.2. Literature Review
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Scope Planning
3.2. Hazard Identification
3.2.1. Toxicokinetics
3.2.2. Toxicodynamics
3.2.3. Carcinogenicity Classification
3.2.4. Detection in Tested Devices
3.3. Dose-Response Assessment
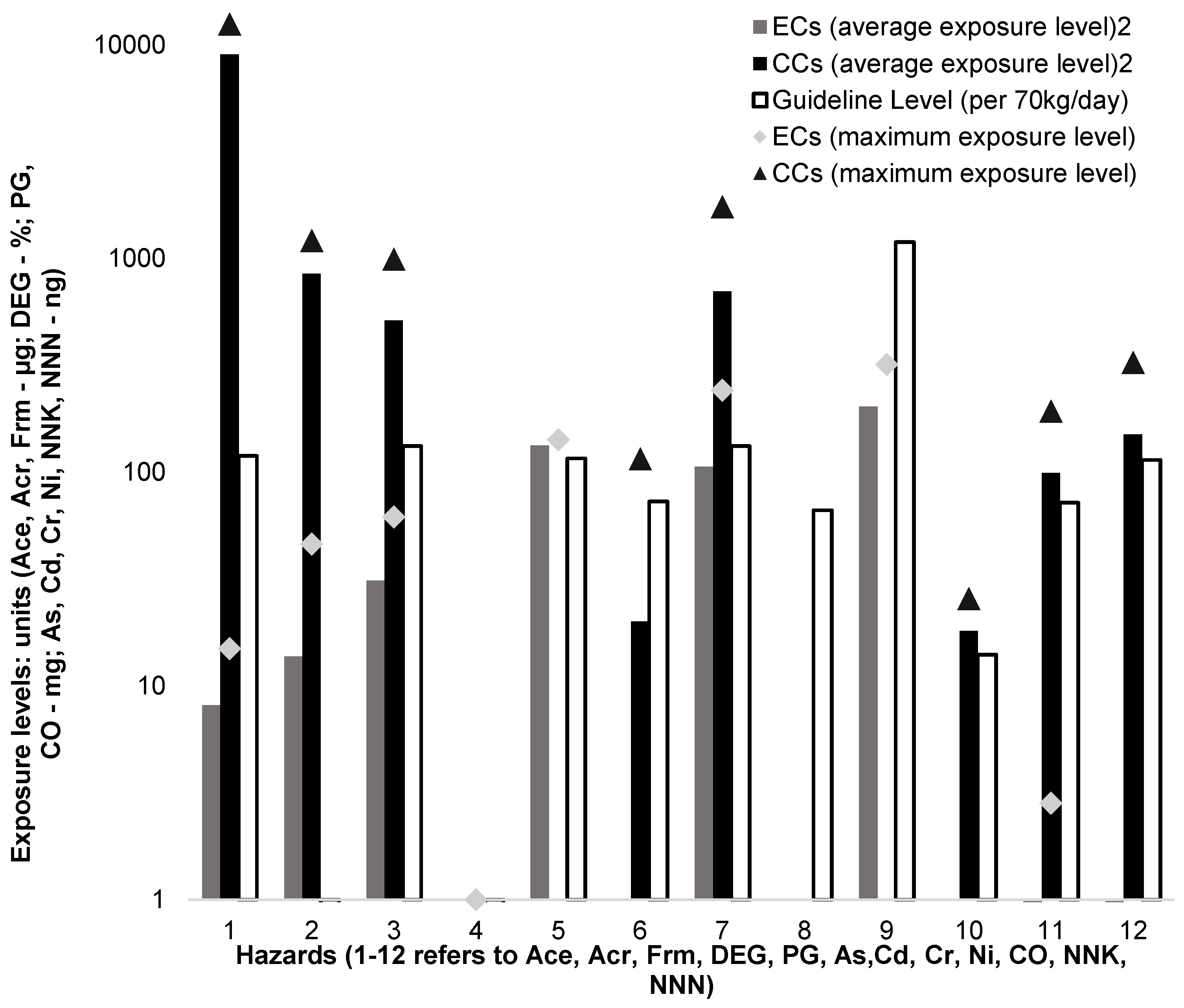
3.4. Exposure Assessment
3.5. Risk Characterisation
3.6. Risk Comparison
4. Discussion
5. Conclusions
5.1. Implications
5.2. Further Research
5.3. Concluding Statement
Author Contributions
Conflicts of Interest
References
- Caponnetto, P.; Campagna, D.; Papale, G.; Russo, C.; Polosa, R. The emerging phenomenon of electronic cigarettes. Expert Rev. Respir. Med. 2012, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Pepper, J.K.; Brewer, N.T. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: A systematic review. Tob. Control 2014, 23, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, C.A.; Gindi, R.M. Electronic Cigarette Use among Adults: United States 2014. Centers for Disease Control and Prevention: United States, 2015. Available online: https://www.cdc.gov/nchs/data/databriefs/db217.pdf (accessed on 13 February 2017).
- Action on Smoking and Health. Use of Electronic Cigarettes (Vapourisers) among Adults in Great Britain. ASH: United Kingdom. 2016. Available online: http://ash.org.uk/information-and-resources/fact-sheets/use-of-electronic-cigarettes-vapourisers-among-adults-in-great-britain/ (accessed on 13 February 2017).
- Electronic Cigarette Statistics. Available online: http://www.statisticbrain.com/electronic-cigarette-statistics/ (accessed on 10 November 2016).
- McNeill, A.; Brose, L.S.; Calder, R.; Hitchman, S.C.; Hajek, P.; McRobbie, H. E-Cigarettes: An Evidence Update (A Report Commissioned by Public Health England). Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457102/Ecigarettes_an_evidence_update_A_report_commissioned_by_Public_Health_England_FINAL.pdf (accessed on 5 January 2016).
- E-Cigarettes: An Emerging Public Health Consensus. Available online: https://www.gov.uk/government/news/e-cigarettes-an-emerging-public-health-consensus (accessed on 5 January 2016).
- United States Food and Drug Administration. Vaporizers, E-Cigarettes, and Other Electronic Nicotine Delivery Systems (ENDS). Available online: http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm172906.htm (accessed on 20 December 2015).
- England, L.; Lisko, J.G.; Pappas, R.S. Important Considerations for Providers Regarding the Use of Electronic Cigarettes. Available online: https://clinmedjournals.org/articles/ijrpm/international-journal-of-respiratory-and-pulmonary-medicine-ijrpm-2-035e.pdf (accessed on 7 March 2017).
- International Programme on Chemical Safety. IPCS Risk Assessment Terminology. Available online: http://www.who.int/ipcs/methods/harmonization/areas/ipcsterminologyparts1and2.pdf (accessed on 1 August 2015).
- United States Environmental Protection Agency. Human Health Risk Assessment. Available online: http://www.epa.gov/riskassessment/health-risk.htm (accessed on 20 December 2015).
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Counts, M.E.; Morton, M.J.; Laffoon, S.W.; Cox, R.H.; Lipowicz, P.J. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005, 41, 185–227. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.V.; O’Connor, R.J.; Stephens, W.E.; Cummings, K.M.; Fong, G.T. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: Results from the International Tobacco Control (ITC) United States survey cohort. Int. J. Environ. Res. Public Health 2014, 11, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. ToxGuide for Carbon Monoxide. Available online: http://www.atsdr.cdc.gov/toxguides/toxguide-201.pdf (accessed on 5 January 2016).
- World Health Organisation. Electronic Nicotine Delivery Systems (Report by WHO). Available online: http://apps.who.int/gb/fctc/PDF/cop6/FCTC_COP6_10Rev1-en.pdf?ua=1 (accessed on 5 January 2016).
- World Health Organisation. Fact Sheet on Ingredients in Tobacco Products. Available online: http://apps.who.int/iris/bitstream/10665/152661/1/WHO_NMH_PND_15.2_eng.pdf?ua=1&ua=1 (accessed on 6 August 2015).
- World Health Organisation. Air Quality Guidelines for Europe, 2nd ed. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/74732/E71922.pdf (accessed on 20 September 2015).
- Agency for Toxic Substances and Disease Registry. Toxicology Curriculum for Communities Trainer’s Manual. Available online: http://www.atsdr.cdc.gov/training/toxmanual/pdf/module-2.pdf (accessed on 20 September 2015).
- Centers for Disease Control and Prevention. Health Effects of Cigarette Smoking. Available online: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/ (accessed on 1 October 2015).
- New Zealand Ministry of Health. Tobacco Use 2012/13: New Zealand Health Survey. Available online: http://www.health.govt.nz/system/files/documents/publications/tobacco-use-2012-13-new-zealand-health-survey-dec14-v2.pdf (accessed on 4 October 2015).
- New Zealand Ministry of Health. Tobacco Returns 2013 Analysis Table. Available online: http://www.health.govt.nz/system/files/documents/pages/2013-tables-tobacco-team.xls (accessed on 13 February 2017).
- Scientific Committee on Consumer Products. Opinion on Diethylene Glycol. Health & Consumer Protection Directorate-General: 2008. Available online: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_139.pdf (accessed on 5 August 2015).
- United States Environmental Protection Agency. Health and Environmental Effects Document for Propylene Glycol. Available online: http://nepis.epa.gov/Exe/ZyPDF.cgi/9100RGZI.PDF?Dockey=9100RGZI.PDF (accessed on 5 August 2015).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Cadmium. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0141_summary.pdf (accessed on 29 July 2015).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Nickel Refinery Dust. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0272_summary.pdf (accessed on 29 July 2015).
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Acrolein. Available online: http://www.atsdr.cdc.gov/ToxProfiles/tp124.pdf (accessed on 20 July 2015).
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Formaldehyde. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp111.pdf (accessed on 20 July 2016).
- Agency for Toxic Substances and Disease Registry. ToxGuide for Arsenic. Available online: http://www.atsdr.cdc.gov/toxguides/toxguide-2.pdf (accessed on 21 July 2015).
- Agency for Toxic Substances and Disease Registry. ToxGuide for Cadmium. Available online: http://www.atsdr.cdc.gov/toxguides/toxguide-5.pdf (accessed on 21 July 2015).
- International Agency for Research on Cancer. Arsenic, Metals, Fibres, and Dusts. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C.pdf (accessed on 6 March 2017).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Acetaldehyde. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0290_summary.pdf (accessed on 28 July 2015).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Acrolein. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0364_summary.pdf (accessed on 28 July 2015).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Formaldehyde. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0419_summary.pdf (accessed on 28 July 2015).
- Agency for Toxic Substances and Disease Registry. Minimal Risk Levels (MRLs). Available online: http://www.atsdr.cdc.gov/mrls/pdfs/atsdr_mrls.pdf (accessed on 5 August 2015).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Arsenic, Inorganic. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0278_summary.pdf (accessed on 30 July 2015).
- United States Environmental Protection Agency Integrated Risk Information System. Chemical Assessment Summary—Chromium (VI). Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0144_summary.pdf (accessed on 30 July 2015).
- California Environmental Protection Agency Office of Environmental Health Hazard Assessment. Proposition 65 Status Report Safe Harbor Levels: No Significant Risk levels for Carcinogens and Maximum Allowable Dose Levels for Chemicals Causing Reproductive Toxicity. Available online: http://oehha.ca.gov/prop65/pdf/June2004StatusRpt.pdf (accessed on 20 August 2015).
- World Health Organisation. The Scientific Basis of Tobacco Product Regulation. Available online: http://www.who.int/tobacco/global_interaction/tobreg/9789241209458.pdf (accessed on 15 August 2015).
- California Environmental Protection Agency Office of Environmental Health Hazard Assessment. Toxicity Criteria Database—OEHHA Cancer Potency Values. Available online: http://oehha.ca.gov/risk/pdf/tcdb072109alpha.pdf (accessed on 20 August 2015).
- New Zealand Ministry for the Environment. Ambient Air Quality Guidelines—2002 Update. Available online: https://www.mfe.govt.nz/sites/default/files/ambient-guide-may02.pdf (accessed on 10 August 2015).
- United States Food and Drug Administration. Testing of Glycerin for Diethylene Glycol. Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070347.pdf (accessed on 5 January 2016).
- Geiss, O.; Bianchi, I.; Barahona, F.; Barrero-Moreno, J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int. J. Hyg. Environ. Health 2015, 218, 169–180. [Google Scholar] [CrossRef] [PubMed]
| Hazard | Cancer Risk | Source Agency | Threshold Level | Source Agency |
|---|---|---|---|---|
| Acetaldehyde | IUCR = 2.2 × 10−6 | IRIS [32] | CIE (RfC) = 9 × 10−3 mg/m3 | IRIS [32] |
| Acrolein | CIE (RfC) = 2 × 10−5 mg/m3 | IRIS [33] | ||
| Formaldehyde | IUCR = 1.3 × 10−5 | IRIS [34] | CIE (MRL) = 9.8 × 10−3 mg/m3 | ATSDR [35] |
| DEG | RfD = 21 mg/70 kg adult/day | SCCP [23] | ||
| PG | CIE (RfD) = 116 mg/70 kg adult/day | U.S. EPA [24] | ||
| As | IUCR = 4.3 × 10−3 | IRIS [36] | CIE = 0.0055 μg/m3 | MFE NZ |
| Cd | IUCR = 1.8 × 10−3 | IRIS [25] | CIE (MRL) = 10 ng/m3 | ATSDR [35] |
| Cr (VI) | IUCR = 1.2 × 10−2 | IRIS [37] | CIE (RfC) = 0.0001 mg/m3 | IRIS [37] |
| Ni | IUCR = 2.4 × 10−4 | IRIS [26] | CIE (MRL) = 90 ng/m3 | ATSDR [35] |
| CO | 15 min RfC = 100 mg/m3 | WHO [18] | ||
| NNK | IUCR = 1.43 × 10−3 | CEPA [38] | RL < 72 ng per mg | WHO [39] |
| NNN | IUCR = 4 × 10−4 | CEPA [40] | RL < 114 ng per mg | WHO [39] |
| Hazards | Guideline Levels | Source Agency |
|---|---|---|
| Acetaldehyde | CIE RfD = 119.25 μg/70 kg/day | IRIS [32] |
| Acrolein | CIE RfD = 0.27 μg/70 kg/day | IRIS [33] |
| Formaldehyde | CIE RfD = 132.50 μg/70 kg/day | ATSDR [35] |
| DEG | <0.1% as e-liquid impurities | SCCP [23] |
| PG | CIE RfD = 116.00 mg/70 kg/day | US EPA [24] |
| As | CIE RfD = 72.88 ng/70 kg/day | MFE NZ [41] |
| Cd | CIE RfD = 132.50 ng/70 kg/day | ATSDR [35] |
| Cr | CIE RfD = 66.25 ng/70 kg/day | ATSDR [35] |
| Ni | CIE RfD = 1192.50 ng/70 kg/day | ATSDR [35] |
| CO | 15 min RfD = 14.00 mg/70 kg for 15 min | WHO [18] |
| NNK | 72.00 ng/cigarette or 72.00 ng/vaping session | WHO [39] |
| NNN | 114.00 ng/cigarette or 114.00 ng/vaping session | WHO [39] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Bullen, C.; Dirks, K. A Comparative Health Risk Assessment of Electronic Cigarettes and Conventional Cigarettes. Int. J. Environ. Res. Public Health 2017, 14, 382. https://doi.org/10.3390/ijerph14040382
Chen J, Bullen C, Dirks K. A Comparative Health Risk Assessment of Electronic Cigarettes and Conventional Cigarettes. International Journal of Environmental Research and Public Health. 2017; 14(4):382. https://doi.org/10.3390/ijerph14040382
Chicago/Turabian StyleChen, Jinsong, Chris Bullen, and Kim Dirks. 2017. "A Comparative Health Risk Assessment of Electronic Cigarettes and Conventional Cigarettes" International Journal of Environmental Research and Public Health 14, no. 4: 382. https://doi.org/10.3390/ijerph14040382
APA StyleChen, J., Bullen, C., & Dirks, K. (2017). A Comparative Health Risk Assessment of Electronic Cigarettes and Conventional Cigarettes. International Journal of Environmental Research and Public Health, 14(4), 382. https://doi.org/10.3390/ijerph14040382







