Towards a Long-Term Strategy for Voluntary-Based Internal Radiation Contamination Monitoring: A Population-Level Analysis of Monitoring Prevalence and Factors Associated with Monitoring Participation Behavior in Fukushima, Japan
Abstract
:1. Introduction
2. Materials and Methods
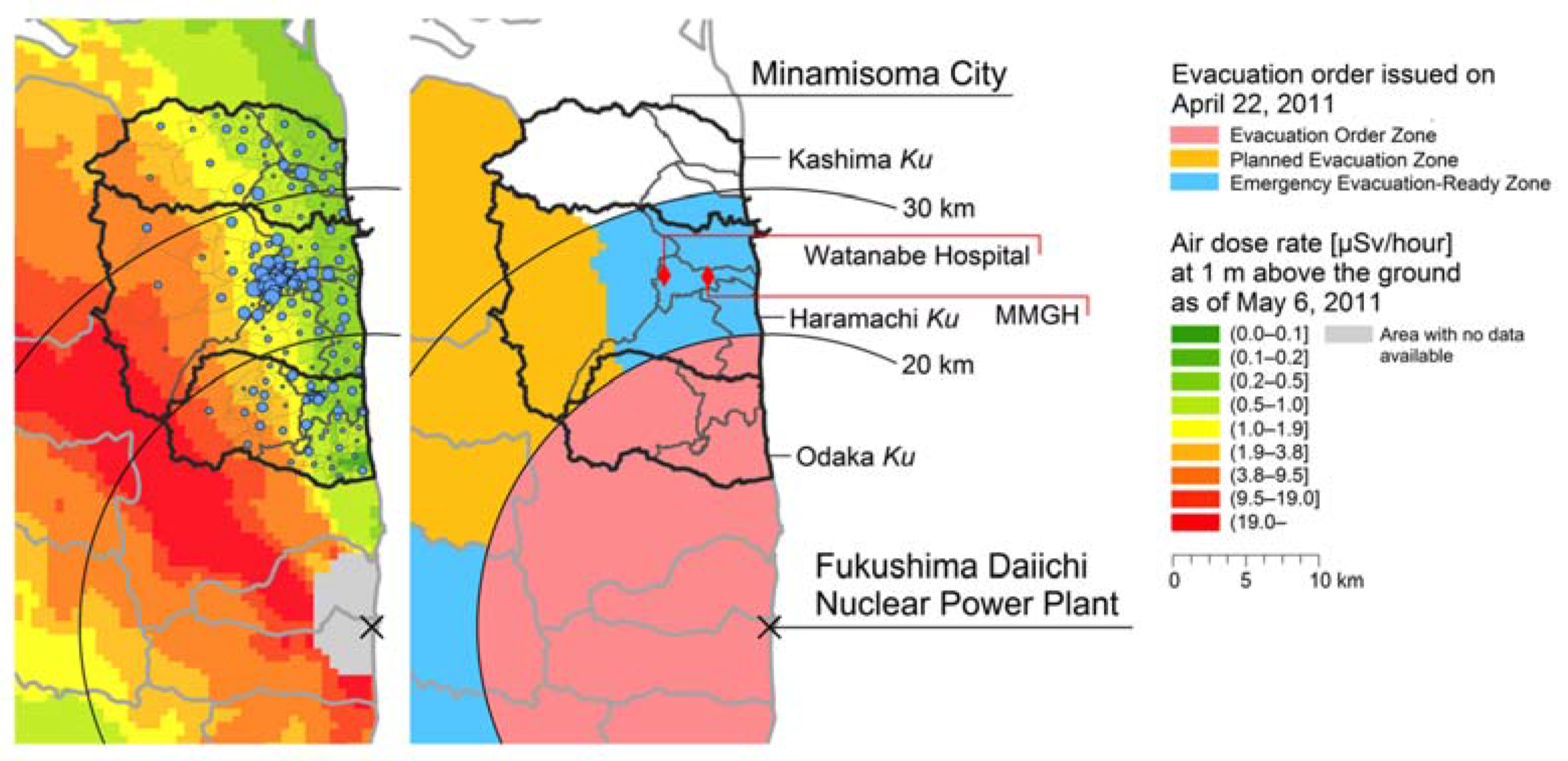
2.1. Setting
2.2. Data Collection
2.2.1. Whole Body Counter Database
2.2.2. Definition of the ‘WBC Monitoring Prevalence’
2.2.3. Evacuation Database
2.3. Eligibility Criteria of Study Subjects
2.4. Data Analysis
2.4.1. Analysis 1: Relative Prevalence of WBC Monitoring in 2013–2014 vs. 2011–2012
2.4.2. Analysis 2: Factors Associated with the WBC Monitoring Prevalence in 2011–2012 and 2013–2014
Regression Model
Variables Considered
2.4.3. Analysis 3: Factors Associated with the WBC Monitoring Participation Behavior
Regression Model
Variables Considered
Sub-Analysis for the Consideration of ‘Being Detected’ with Cs
2.5. Ethics Approval
3. Results
3.1. Demographic Characteristics
3.2. Analysis 1: Relative Prevalence of WBC Monitoring in 2013–2014 vs. 2011–2012
3.3. Analysis 2: Factors Associated with the WBC Monitoring Prevalence in 2011–2012 and 2013–2014
3.4. Analysis 3: Factors Associated with the WBC Monitoring Behavior
4. Discussion
4.1. Radiation Risk Perception
4.1.1. Internal Contamination Monitoring as a Risk Communication Tool
4.2. Accessibility to the Monitoring
4.3. Late-Stage Elderly Cohorts
4.4. Future Recommendations
- (1)
- Accessibility to the monitoring program (in terms of distance and operating times) may influence the monitoring participation behavior—there may exist some people who want to participate in the monitoring, but are not able to do so due to reasons beyond their personal control (e.g., difficult work schedules, limited transportation, mobility due to health conditions, etc.), indicating a gap in the monitoring delivery between the supply-side (monitoring providers) and demand-side (participants). In order to minimize this gap, the monitoring providers should consider, as one solution, the use of a mobile WBC unit (i.e., a WBC unit installed in a bus-like trailer for easy transportation), which can move around the city, and operate over a wider range of hours, to reduce the access barrier for these participants.
- (2)
- Radiation risk perception may influence monitoring participation behavior, and thus people who are concerned about radiation exposure might be more likely to participate in voluntary-based monitoring. Since radiation risk perception also associates with dietary consumption behavior [50], monitoring results based on voluntary participation may not represent the exposure levels of the general population. Policymakers and implementers as well as researchers should carefully consider the potential for and effects of this bias. Note that because the internal contamination level has been very limited in Minamisoma City [9,12], this bias is likely to be minimal.
- (3)
- The function of internal contamination monitoring should not be considered simply as informing personal and public health measures to ensure internal contamination levels are sufficiently low. Monitoring may also function as a risk communication tool, alleviating individuals’ concern and anxiety over radiation contamination and enhancing their well-being; however, this remains conjecture. Rigorous investigation is, therefore, needed to evaluate if, how much, and in what way the internal contamination monitoring can work so.
4.5. Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- UNSCEAR. Effects of Ionizing Radiation: UNSCEAR 2006 Report to the General Assembly, with Scientific Annexes; United Nations Scientific Committee on the Effects of Atomic Radiation: New York, NY, USA, 2008. [Google Scholar]
- Nakayachi, K.; Nagaya, K. The effects of the passage of time from the 2011 Tohoku earthquake on the public's anxiety about a variety of hazards. Int. J. Environ. Res. Public Health 2016, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Brumfiel, G. Fukushima: Fallout of fear. Nature 2013, 493, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Tsubokura, M.; Furutani, T.; Hayano, R.S.; Kami, M.; Kanazawa, Y.; Oikawa, T. Dependence of radiation dose on the behavioral patterns among school children: A retrospective analysis 18 to 20 months following the 2011 Fukushima nuclear incident in Japan. J. Radiat. Res. 2016, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsubokura, M.; Nomura, S.; Sakaihara, K.; Kato, S.; Leppold, C.; Furutani, T.; Morita, T.; Oikawa, T.; Kanazawa, Y. Estimated association between dwelling soil contamination and internal radiation contamination levels after the 2011 Fukushima Daiichi nuclear accident in Japan. BMJ Open 2016, 6, e010970. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.; Miyagawa, S.; Kasuga, F.; Shibuya, K. Current measures on radioactive contamination in Japan: A policy situation analysis. PLoS ONE 2016, 11, e0152040. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Ogino, H. Food safety regulations: What we learned from the Fukushima nuclear accident. J. Environ. Radioact. 2012, 111, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Takanashi, Y.; Kihara, A.; Tsutake, T.; Mitsui, Y. Radiation screening test of commercial food products and foodstuffs for food services using Nal (T1) scintillation survey meter. J. Food Hyg. Soc. Jpn. 2013, 54, 127–130. [Google Scholar] [CrossRef]
- Tsubokura, M.; Gilmour, S.; Takahashi, K.; Oikawa, T.; Kanazawa, Y. Internal radiation exposure after the Fukushima nuclear power plant disaster. J. Am. Med. Assn. 2012, 308, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Gilmour, S.; Tsubokura, M.; Nomura, S.; Kami, M.; Oikawa, T.; Kanazawa, Y.; Shibuya, K. Assessment of the risk of medium-term internal contamination in Minamisoma City, Fukushima, Japan, after the Fukushima Dai-ichi nuclear accident. Environ. Health Perspect. 2014, 122, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Hayano, R.S.; Tsubokura, M.; Miyazaki, M.; Satou, H.; Sato, K.; Masaki, S.; Sakuma, Y. Internal radiocesium contamination of adults and children in Fukushima 7 to 20 months after the Fukushima NPP accident as measured by extensive whole-body-counter surveys. Proc. Jpn. Acad. Ser. B, Phys.Biol. Sci. 2013, 89, 157–163. [Google Scholar] [CrossRef]
- Tsubokura, M.; Kato, S.; Morita, T.; Nomura, S.; Kami, M.; Sakaihara, K.; Hanai, T.; Oikawa, T.; Kanazawa, Y. Assessment of the annual additional effective doses amongst minamisoma children during the second year after the fukushima daiichi nuclear power plant disaster. PLoS ONE 2015, 10, e0129114. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency Protective Action Guides (PAGs). Available online: https://www.epa.gov/radiation/protective-action-guides-pags (accessed on 21 February 2017).
- Balonov, M.I.; Anspaugh, L.R.; Bouville, A.; Likhtarev, I.A. Contribution of internal exposures to the radiological consequences of the Chernobyl accident. Radiat. Prot. Dosim. 2007, 127, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hayano, R.S.; Watanabe, Y.N.; Nomura, S.; Nemoto, T.; Tsubokura, M.; Hanai, T.; Kumemoto, Y.; Kowata, S.; Oikawa, T.; Kanazawa, Y. Whole-body counter survey results 4 months after the Fukushima Dai-ichi NPP accident in Minamisoma City, Fukushima. J. Radiol. Prot.: Offic. J. Soc. Radiol. Prot. 2014, 34, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, M.; Konstantinov, Y.O.; Evdeeva, T.Y.; Kovalev, A.I.; Aksenov, A.S.; Koulikova, N.V.; Sato, H.; Takatsui, T.; Takada, J.; Endo, S.; et al. Radiocesium in children residing in the western districts of the Bryansk Oblast from 1991–1996. Health phys. 2000, 79, 182–186. [Google Scholar] [CrossRef] [PubMed]
- DeVita, R.; Olivieri, A.; Spinelli, A.; Grollino, M.G.; Padovani, L.; Tarroni, G.; Cozza, R.; Sorcini, M.; Pennelli, P.; Casparrini, G.; et al. Health status and internal radiocontamination assessment in children exposed to the fallout of the Chernobyl accident. Arch. Environ. Health 2000, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bernhardsson, C.; Zvonova, I.; Raaf, C.; Mattsson, S. Measurements of long-term external and internal radiation exposure of inhabitants of some villages of the Bryansk region of Russia after the Chernobyl accident. Sci. Total Envir. 2011, 409, 4811–4817. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Yale University Press: New Haven, CT, USA, 2008. [Google Scholar]
- Midorikawa, S.; Tanigawa, K.; Suzuki, S.; Ohtsuru, A. Psychosocial issues related to thyroid examination after a radiation disaster. Asia-Pac. J. Public Health 2017, 29, 63S–73S. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Tsubokura, M. Evaluating risk communication after the Fukushima disaster based on nudge theory. Asia-Pac. J. Public Health 2017, 29, 193S–200S. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, J. Government ‘nudges’ prove their worth. Science 2016, 352, 1042. [Google Scholar] [CrossRef] [PubMed]
- Minamisoma City Office Population based on basic resident register of Minamisoma City. Available online: http://www.city.minamisoma.lg.jp/index.cfm/8.4705.44.html (accessed 18 October 2016).
- The National Diet of Japan Fukushima Nuclear Accident Independent Investigation Commission Overview of the damage and how it spread. Available online: http://warp.da.ndl.go.jp/info:ndljp/pid/3856371/naiic.go.jp/wp-content/uploads/2012/08/NAIIC_Eng_Chapter4_web.pdf (accessed 18 October 2016).
- ICRP. ICRP Publication 103: Recommendations of the ICRP; International Commission on Radiological Protection: New York, NY, USA, 2013. [Google Scholar]
- Nomura, S.; Tsubokura, M.; Gilmour, S.; Hayano, R.S.; Watanabe, Y.N.; Kami, M.; Kanazawa, Y.; Oikawa, T. An evaluation of early countermeasures to reduce the risk of internal radiation exposure after the Fukushima nuclear incident in Japan. Health Policy Plann. 2016, 3, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education, Culture, Sports, Science and Technology Results of the first airborne monitoring survey. Available online: http://radioactivity.nsr.go.jp/ja/list/362/list-1.html%20 (accessed on 18 October 2016).
- WHO. Health Risk Assessment from the Nuclear Accident after the 2011 Great East. Japan Earthquake and Tsunami, Based on a Preliminary Dose Estimation; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- UNSCEAR. Fukushima 2015 White Paper: Developments since the 2013 UNSCEAR Report on the Levels and Effects of Radiation Exposure due to the Nuclear Accident Following the Great East-Japan Earthquake and Tsunami; United Nations Scientific Committee on the Effects of Atomic Radiation: New York, NY, USA, 2015. [Google Scholar]
- Kim, S.E.; Perez-Stable, E.J.; Wong, S.; Gregorich, S.; Sawaya, G.F.; Walsh, J.M.; Kaplan, C.P. Association between cancer risk perception and screening behavior among diverse women. Arch. Internal Med. 2008, 168, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Holman, L.L.; Lu, K.H.; Bast, R.C., Jr.; Hernandez, M.A.; Bodurka, D.C.; Skates, S.; Sun, C.C. Risk perception, worry, and test acceptance in average-risk women who undergo ovarian cancer screening. Amer. J. Obstet. Gynecol. 2014, 210, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Deary, I.J.; Wardle, J.; Zaninotto, P.; Batty, G.D. Cognitive ability and personality as predictors of participation in a national colorectal cancer screening programme: The English Longitudinal Study of Ageing. J. Epidemiol.Community Health 2015, 69, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Pisera, M.; Kaminski, M.F.; Kraszewska, E.; Rupinski, M.; Regula, J. Reinvitation to screening colonoscopy: A randomized-controlled trial of reminding letter and invitation to educational meeting on attendance in nonresponders to initial invitation to screening colonoscopy (REINVITE). Eur. J. Gastroenterol. Hepatol. 2016, 28, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Senore, C.; Ederle, A.; DePretis, G.; Magnani, C.; Canuti, D.; Deandrea, S.; Zorzi, M.; Barca, A.; Bestagini, P.; Faitini, K.; et al. Invitation strategies for colorectal cancer screening programmes: The impact of an advance notification letter. Prev. Med. 2015, 73, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; von Wagner, C.; Kralj-Hans, I.; Halloran, S.P.; Smith, S.G.; McGregor, L.M.; Vart, G.; Howe, R.; Snowball, J.; Handley, G.; et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): Four cluster-randomised controlled trials. Lancet 2016, 387, 751–759. [Google Scholar] [CrossRef]
- Curtin, R.; Presser, S.; Singer, E. The effects of response rate changes on the index of consumer sentiment. Publ. Opin. Quart. 2000, 64, 413–428. [Google Scholar] [CrossRef]
- Singer, E.; Van Hoewyk, J.; Maher, M.P. Experiments with incentives in telephone surveys. Publ. Opin. Quart. 2000, 64, 171–188. [Google Scholar] [CrossRef]
- Legleye, S.; Bohet, A.; Razafindratsima, N.; Bajos, N.; Fecond Research, T.; Moreau, C. A randomized trial of survey participation in a national random sample of general practitioners and gynecologists in France. Revue d'Epidemiologie et de Sante Publique 2014, 62, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Marcon, A.; Nguyen, G.; Rava, M.; Braggion, M.; Grassi, M.; Zanolin, M.E. A score for measuring health risk perception in environmental surveys. Sci. Total Environ. 2015, 527–528, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yabe, H.; Yasumura, S.; Ohira, T.; Niwa, S.; Ohtsuru, A.; Mashiko, H.; Maeda, M.; Abe, M.; The Mental Health Group of the Fukushima Health Management Survey. Psychological distress and the perception of radiation risks: The Fukushima health management survey. Bull. World Health Organ. 2015, 93, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Hino, Y.; Murakami, M.; Midorikawa, S.; Ohtsuru, A.; Suzuki, S.; Tsuboi, K.; Ohira, T. Explanatory Meetings on Thyroid Examination for the “Fukushima Health Management Survey” after the Great East Japan Earthquake: Reduction of Anxiety and Improvement of Comprehension. Tohoku J. Exp. Med. 2016, 239, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Morioka, R. Gender difference in the health risk perception of radiation from Fukushima in Japan: The role of hegemonic masculinity. Soc. Sci. Med. 2014, 107, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Minamisoma City Office Results of the internal radiation exposure monitoring (9) in Japanese. Available online: https://www.city.minamisoma.lg.jp/index.cfm/10,28760,61,344,html (accessed 18 October 2016).
- Tsubokura, M.; Kato, S.; Nihei, M.; Sakuma, Y.; Furutani, T.; Uehara, K.; Sugimoto, A.; Nomura, S.; Hayano, R.; Kami, M.; et al. Limited internal radiation exposure associated with resettlements to a radiation-contaminated homeland after the Fukushima Daiichi nuclear disaster. PLoS ONE 2013, 8, e81909. [Google Scholar] [CrossRef] [PubMed]
- Tsubokura, M.; Kato, S.; Nomura, S.; Gilmour, S.; Nihei, M.; Sakuma, Y.; Oikawa, T.; Kanazawa, Y.; Kami, M.; Hayano, R. Reduction of high levels of internal radio-contamination by dietary intervention in residents of areas affected by the Fukushima Daiichi nuclear plant disaster: A case series. PLoS ONE 2014, 9, e100302. [Google Scholar] [CrossRef] [PubMed]
- Hayano, R.S.; Yamanaka, S.; Bronson, F.L.; Oginni, B.; Muramatsu, I. BABYSCAN: A whole body counter for small children in Fukushima. J. Radiol. Prot.: Offic. J. Soc. Radiol. Prot. 2014, 34, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Tanigawa, K.; Ohtsuru, A.; Yabe, H.; Maeda, M.; Shigemura, J.; Ohira, T.; Tominaga, T.; Akashi, M.; Hirohashi, N.; et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet 2015, 386, 479–488. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare Labor force participation rate by gender and age group. Available online: http://www.mhlw.go.jp/toukei/itiran/roudou/koyou/doukou/16–2/dl/kekka_gaiyo-03.pdf (accessed on 18 October 2016).
- Pinquart, M.; Duberstein, P.R. Information needs and decision-making processes in older cancer patients. Crc. Crit. Rev. Oncol. Hematol. 2004, 51, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Orita, M.; Hayashida, N.; Nakayama, Y.; Shinkawa, T.; Urata, H.; Fukushima, Y.; Endo, Y.; Yamashita, S.; Takamura, N. Bipolarization of Risk Perception about the Health Effects of Radiation in Residents after the Accident at Fukushima Nuclear Power Plant. PLoS ONE 2015, 10, e0129227. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number (%) |
|---|---|
| Age at 11 March 2011 | |
| 21–30 | 4012 (8.8) |
| 31–40 | 6692 (14.6) |
| 41–50 | 6663 (14.6) |
| 51–60 | 9550 (20.9) |
| 61–70 | 9113 (19.9) |
| 71–80 | 6459 (14.1) |
| ≥81 | 3299 (7.2) |
| Gender | |
| Male | 21929 (47.9) |
| Female | 23859 (52.1) |
| Original residential area by evacuation instruction | |
| Outside the evacuation zones | 6722 (14.7) |
| Evacuation Order Zone | 9471 (20.7) |
| Emergency Evacuation-Ready Zone | 29595 (64.6) |
| Post-incident dwelling area at 1 June 2011 | |
| Inside Minamisoma City | 24926 (51.2) |
| Inside Fukushima/Outside Minamisoma City | 7875 (16.2) |
| Neighboring Prefectures | 7589 (15.6) |
| Outside Fukushima & neighboring Prefectures | 5398 (11.1) |
| Post-incident dwelling area at 1 June 2013 | |
| Inside Minamisoma City | 36074 (74.2) |
| Inside Fukushima/Outside Minamisoma City | 4107 (8.4) |
| Neighboring Prefectures | 3437 (7.1) |
| Outside Fukushima & neighboring Prefectures | 2170 (4.5) |
| 2011–2012 | 2013–2014 | RP (95% Confidence Interval) | |
|---|---|---|---|
| Male | |||
| Age at 11 March 2011 | |||
| 21–30 | 22.1 (481) | 7.2 (120) | 0.33 (0.27–0.40) *** |
| 31–40 | 28.6 (983) | 11.1 (362) | 0.39 (0.35–0.43) *** |
| 41–50 | 28.0 (940) | 11.5 (384) | 0.41 (0.37–0.46) *** |
| 51–60 | 26.4 (1253) | 14.2 (604) | 0.54 (0.49–0.59) *** |
| 61–70 | 31.7 (1433) | 22.4 (1110) | 0.71 (0.66–0.75) *** |
| 71–80 | 28.0 (747) | 24.5 (737) | 0.88 (0.80–0.96) ** |
| ≥81 | 13.6 (138) | 12.6 (181) | 0.93 (0.75–1.13) |
| Total | 27.2 (5975) | 16.0 (3498) | 0.59 (0.56–0.61) *** |
| Female | |||
| Age at 11 March 2011 | |||
| 21–30 | 35.9 (658) | 19.3 (264) | 0.54 (0.47–0.61) *** |
| 31–40 | 41.2 (1341) | 17.8 (540) | 0.43 (0.40–0.47) *** |
| 41–50 | 37.5 (1239) | 18.0 (582) | 0.48 (0.44–0.52) *** |
| 51–60 | 36.8 (1767) | 22.6 (987) | 0.62 (0.58–0.66) *** |
| 61–70 | 36.9 (1692) | 27.4 (1356) | 0.74 (0.70–0.79) *** |
| 71–80 | 24.6 (931) | 19.7 (767) | 0.80 (0.74–0.87) *** |
| ≥81 | 9.4 (215) | 6.2 (187) | 0.66 (0.55–0.80) *** |
| Total | 32.9 (7843) | 19.6 (4683) | 0.60 (0.58–0.62) *** |
| Overall | 30.2 (13818) | 17.9 (8181) | 0.59 (0.58–0.61) *** |
| 2011–2012 | 2013–2014 | |
|---|---|---|
| Age at 11 March 2011 | ||
| 21–30 | 1.00 | 1.00 |
| 31–40 | 1.21 (1.09–1.35) *** | 1.14 (0.92–1.40) |
| 41–50 | 1.10 (0.99–1.22) | 1.12 (0.91–1.38) |
| 51–60 | 1.06 (0.96–1.18) | 1.47 (1.19–1.81) *** |
| 61–70 | 1.15 (1.04–1.28) ** | 1.80 (1.47–2.21) *** |
| 71–80 | 0.86 (0.77–0.96) ** | 1.64 (1.32–2.03) *** |
| ≥81 | 0.35 (0.30–0.41) *** | 0.59 (0.46–0.75) *** |
| Gender | ||
| Male | 1.00 | 1.00 |
| Female | 1.25 (1.18–1.32) *** | 1.32 (1.19–1.47) *** |
| Original residential area by evacuation instruction | ||
| Outside the evacuation zones | 1.00 | 1.00 |
| Evacuation Order Zone | 1.78 (1.57–2.03) *** | 1.45 (1.20–1.73) *** |
| Emergency Evacuation-Ready Zone | 1.51 (1.36–1.68) *** | 1.31 (1.10–1.56) ** |
| Post-incident dwelling area | ||
| Inside Minamisoma City | 1.00 | 1.00 |
| Inside Fukushima/Outside Minamisoma City | 0.99 (0.81–1.21) | 1.05 (0.68–1.61) |
| Neighboring Prefectures | 0.97 (0.83–1.20) | 0.64 (0.38–1.09) |
| Outside Fukushima & neighboring Prefectures | 0.92 (0.71–1.19) | 0.33 (0.14–0.81) * |
| Original Residential Area by Evacuation Instruction | Post-Incident Dwelling Area | 2011–2012 | 2013–2014 |
|---|---|---|---|
| Outside the evacuation zones | Inside Minamisoma City | 1.00 | 1.00 |
| Inside Fukushima/Outside Minamisoma City | 0.99 (0.81–1.21) | 1.05 (0.68–1.61) | |
| Neighboring Prefectures | 1.00 (0.83–1.20) | 0.64 (0.38–1.09) | |
| Outside Fukushima & neighboring Prefectures | 0.92 (0.71–1.19) | 0.33 (0.14–0.81) * | |
| Evacuation Order Zone | Inside Minamisoma City | 1.00 | 1.00 |
| Inside Fukushima/Outside Minamisoma City | 0.84 (0.74–0.95) ** | 0.66 (0.54–0.82) *** | |
| Neighboring Prefectures | 0.64 (0.56–0.73) *** | 0.29 (0.22–0.39) *** | |
| Outside Fukushima & neighboring Prefectures | 0.48 (0.41–0.56) *** | 0.12 (0.08–0.19) *** | |
| Emergency Evacuation-Ready Zone | Inside Minamisoma City | 1.00 | 1.00 |
| Inside Fukushima/Outside Minamisoma City | 0.98 (0.88–1.09) | 0.84 (0.69–1.02) | |
| Neighboring Prefectures | 0.78 (0.70–0.87) *** | 0.56 (0.45–0.69) *** | |
| Outside Fukushima & neighboring Prefectures | 0.69 (0.61–0.77) *** | 0.45 (0.35–0.58) *** |
| Pattern (I) | Pattern (II) | Pattern (III) | Pattern (IV) (non-participation) | |
|---|---|---|---|---|
| Male | ||||
| Age at 11 March 2011 | ||||
| 21–30 | 3.8 (83) | 18.2 (398) | 3.5 (76) | 74.5 (1624) |
| 31–40 | 7.1 (244) | 21.5 (739) | 4.7 (161) | 66.7 (2294) |
| 41–50 | 6.7 (225) | 21.3 (715) | 4.9 (163) | 67.1 (2253) |
| 51–60 | 7.9 (377) | 18.5 (876) | 7.9 (375) | 65.7 (3118) |
| 61–70 | 12.9 (583) | 18.8 (850) | 10.7 (484) | 57.6 (2606) |
| 71–80 | 11.5 (307) | 16.5 (440) | 11.3 (301) | 60.8 (1624) |
| ≥81 | 5.1 (52) | 8.5 (86) | 6.6 (67) | 79.8 (808) |
| Total | 8.5 (1871) | 18.7 (4104) | 7.4 (1627) | 65.3 (14327) |
| Female | ||||
| Age at 11 March 2011 | ||||
| 21–30 | 10.6 (194) | 25.3 (464) | 8.1 (148) | 56.0 (1025) |
| 31–40 | 10.6 (344) | 30.6 (997) | 7.3 (237) | 51.5 (1676) |
| 41–50 | 10.7 (353) | 26.8 (886) | 7.3 (241) | 55.2 (1827) |
| 51–60 | 12.9 (619) | 23.9 (1148) | 11.7 (562) | 51.5 (2475) |
| 61–70 | 15.0 (690) | 21.8 (1002) | 11.9 (545) | 51.3 (2353) |
| 71–80 | 8.4 (319) | 16.2 (612) | 8.5 (321) | 66.9 (2535) |
| ≥81 | 2.3 (53) | 7.1 (162) | 2.5 (57) | 88.1 (2014) |
| Total | 10.8 (2572) | 22.1 (5271) | 8.8 (2111) | 58.3 (13,905) |
| Overall | 9.7 (4443) | 20.5 (9375) | 8.2 (3738) | 61.7 (28,232) |
| Pattern (I) | Pattern (II) | Pattern (III) | |
|---|---|---|---|
| Age at 11 March 2011 | |||
| 21–30 | 1.00 | 1.00 | 1.00 |
| 31–40 | 1.41 (1.21–1.64) *** | 1.34 (1.22–1.48) *** | 1.18 (0.99–1.40) |
| 41–50 | 1.28 (1.10–1.49) ** | 1.16 (1.05–1.28) ** | 1.15 (0.97–1.36) |
| 51–60 | 1.55 (1.34–1.79) *** | 1.04 (0.94–1.14) | 1.91 (1.64–2.23) *** |
| 61–70 | 2.25 (1.95–2.58) *** | 1.08 (0.99–1.19) | 2.35 (2.02–2.74) *** |
| 71–80 | 1.24 (1.07–1.44) ** | 0.70 (0.63–0.78) *** | 1.63 (1.39–1.92) *** |
| ≥81 | 0.28 (0.23–0.36) *** | 0.22 (0.19–0.26) *** | 0.45 (0.36–0.57) *** |
| Gender | |||
| Male | 1.00 | 1.00 | 1.00 |
| Female | 1.56 (1.46–1.66) *** | 1.48 (1.41–1.55) *** | 1.41 (1.32–1.52) *** |
| Original residential area by evacuation instruction | |||
| Outside the evacuation zones | 1.00 | 1.00 | 1.00 |
| Evacuation Order Zone | 3.17 (2.70–3.71) *** | 2.03 (1.82–2.27) *** | 1.27 (1.09–1.48) ** |
| Emergency Evacuation-Ready Zone | 1.88 (1.69–1.09) *** | 1.47 (1.36–1.58) *** | 1.03 (0.93–1.14) |
| Evacuation history (beyond Ku) | |||
| History (a) | 0.45 (0.40–0.51) *** | 0.58 (0.54–0.63) *** | 0.74 (0.65–0.83) *** |
| History (b) | 1.06 (0.97–1.15) | 0.96 (0.90–1.03) | 1.18 (1.08–1.29) *** |
| History (c) | 0.89 (0.74–1.07) | 1.00 (0.88–1.14) | 1.17 (0.96–1.43) |
| History (d) (non-evacuation) | 1.00 | 1.00 | 1.00 |
| Air dose rate [μSv/h] as of 22 April 2011 at original residential area | 1.17 (1.15–1.19) *** | 1.21 (1.19–1.23) *** | 1.07 (1.04–1.10) *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, S.; Tsubokura, M.; Ozaki, A.; Murakami, M.; Hodgson, S.; Blangiardo, M.; Nishikawa, Y.; Morita, T.; Oikawa, T. Towards a Long-Term Strategy for Voluntary-Based Internal Radiation Contamination Monitoring: A Population-Level Analysis of Monitoring Prevalence and Factors Associated with Monitoring Participation Behavior in Fukushima, Japan. Int. J. Environ. Res. Public Health 2017, 14, 397. https://doi.org/10.3390/ijerph14040397
Nomura S, Tsubokura M, Ozaki A, Murakami M, Hodgson S, Blangiardo M, Nishikawa Y, Morita T, Oikawa T. Towards a Long-Term Strategy for Voluntary-Based Internal Radiation Contamination Monitoring: A Population-Level Analysis of Monitoring Prevalence and Factors Associated with Monitoring Participation Behavior in Fukushima, Japan. International Journal of Environmental Research and Public Health. 2017; 14(4):397. https://doi.org/10.3390/ijerph14040397
Chicago/Turabian StyleNomura, Shuhei, Masaharu Tsubokura, Akihiko Ozaki, Michio Murakami, Susan Hodgson, Marta Blangiardo, Yoshitaka Nishikawa, Tomohiro Morita, and Tomoyoshi Oikawa. 2017. "Towards a Long-Term Strategy for Voluntary-Based Internal Radiation Contamination Monitoring: A Population-Level Analysis of Monitoring Prevalence and Factors Associated with Monitoring Participation Behavior in Fukushima, Japan" International Journal of Environmental Research and Public Health 14, no. 4: 397. https://doi.org/10.3390/ijerph14040397





