Comparison of Biogenic Amines and Mycotoxins in Alfalfa and Red Clover Fodder Depending on Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dolezal, P.; Dvoracek, J.; Loucka, R.; Mikyska, F.; Mudrik, Z.; Boberfeld, O.; Prokes, K.; Prikryl, J.; Skladanka, J.; Strakova, E.; et al. Konzervace Krmiv a Jejich Využití ve Výživě Zvířat, 1st ed.; Vydavatelství Petr Baštan: Olomouc, Czech Republic, 2012; p. 308. [Google Scholar]
- Seppala, A.; Heikkila, T.; Maki, M.; Miettinen, H.; Rinne, M. Controlling aerobic stability of grass silage-based total mixed rations. Anim. Feed Sci. Technol. 2013, 179, 54–60. [Google Scholar] [CrossRef]
- Scherer, R.; Gerlach, K.; Sudekum, K.H. Biogenic amines and gamma-amino butyric acid in silages: Formation, occurrence and influence on dry matter intake and ruminant production. Anim. Feed Sci. Technol. 2015, 210, 1–16. [Google Scholar] [CrossRef]
- Santos, S.M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Nishino, N.; Hattori, H.; Wada, H.; Touno, E. Biogenic amine production in grass, maize and total mixed ration silages inoculated with Lactobacillus casei or Lactobacillus buchneri. J. Appl. Microbiol. 2007, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Selwet, M.; Galbas, M.; Porzucek, F.; Koczorowski, M.; Pociejowska, M. Effects of the method of alfalfa ensiling on the content of biogenic amines and numbers of some strains of Lactobacillus spp. Med. Weter. 2013, 69, 358–362. [Google Scholar]
- Steidlova, S.; Kalac, P. Biogenic amines in grass and clover-grass silages. Collect. Sci. Papers Fac. Agric. Ceske Budejovice Ser. Anim. Sci. 2002, 19, 153–160. [Google Scholar]
- Steidlova, S.; Kalac, P. Levels of biogenic amines in maize silages. Anim. Feed Sci. Technol. 2002, 102, 197–205. [Google Scholar] [CrossRef]
- Skladanka, J.; Nedelnik, J.; Aadam, V.; Dolezal, P.; Moravcova, H.; Dohnal, V. Forage as a primary source of mycotoxins in animal diets. Int. J. Environ. Res. Public Health 2011, 8, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Gravesen, S.; Frisvad, J.C.; Samson, R.A. Microfungi; Munksgaard: Copenhagen, Denmark, 1994; p. 168. [Google Scholar]
- Lillehoj, E.B.; Diener, U.L.; Asquith, R.L.; Dickens, J.W. Aflatoxin and Aspergillus Flavus in Corn. In Southern Cooperative Series Bulletin; Kentucky Agricultural Experiment Station: Lexington, KY, USA, 1983; Volume 279, p. 27. [Google Scholar]
- Vasatkova, A.; Krizova, S.; Krystofova, O.; Adam, V.; Zeman, L.; Beklova, M.; Kizek, R. Effect of naturally mouldy wheat or fungi administration on metallothioneins-3 level in brain tissues of rats. Neuroendocrinol. Lett. 2010, 30, 163–168. [Google Scholar]
- Dohnal, V.; Jezkova, A.; Jun, D.; Kuca, K. Metabolic pathways of T-2 toxin. Curr. Drug Metab. 2008, 9, 77–82. [Google Scholar] [PubMed]
- Hartman, M. Stanovení neutrálnmích těkavých látek v silážích a senážích plynovou chromatografií. Živočišná Výroba 1974, 4, 209–216. [Google Scholar]
- Driehuis, F.; Elferink Oude, S.J.W.H. The impact of the quality of silage on animal health and food safety: A review. Vet. Quart. 2000, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Gollop, N.; Zakin, V.; Weinberg, Z.G. Antibacterial activity of lactic acid bacteria included in inoculants for silage and in silages treated with these inoculants. J. Appl. Microbiol. 2005, 98, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Van Os, M.; Van Wikselaar, P.G.; Spoelstra, S.F. Formation of biogenic amines in well fermented grass silages. J. Agric. Sci. 1996, 127, 97–107. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Hugas, M.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Reduction of biogenic amine formation using a negative amino acid-decarboxylase starter culture for fermentation of fuet sausage. J. Food Prot. 2000, 63, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tveit, B.; Lingaas, F.; Svendsen, M.; Sjaastad, O.V. Etiology of acetonemia in Norwegian cattle. 1. Effect of ketogenic silage, season, energy level, and genetic factors. J. Dairy Sci. 1992, 75, 2421–2432. [Google Scholar] [CrossRef]
- Steidlova, S.; Kalac, P. The effects of lactic acid bacteria inoculants and formic acid on the formation of biogenic amines in grass silages. Arch. Anim. Nutr. 2004, 58, 245–254. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Macpherson, H.T.; Watt, J.A. The effect of histamine on silage dry-matter intake. Grass Forage Sci. 1963, 18, 230–232. [Google Scholar] [CrossRef]
- Okamoto, M.; Waldo, D.R.; Miller, R.W.; Moore, L.A. Histamine levels in forages and dry matter intake of heifers. J. Dairy Sci. 1964, 47, 1231–1236. [Google Scholar] [CrossRef]
- Holzer, M.; Mayrhuber, E.; Danner, H.; Braun, R. The role of Lactobacillus buchneri in forage preservation. Trends Biotechnol. 2003, 21, 282–287. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.A.; Livesey, C.T. Occurrence and significance of mycotoxins in forage crops and silage: A review. J. Sci. Food Agric. 1998, 77, 1–17. [Google Scholar] [CrossRef]
- Alonso, V.A.; Pereyra, C.M.; Keller, L.A.M.; Dalcero, A.M.; Rosa, C.A.R.; Chiacchiera, S.M.; Cavaglieri, L.R. Fungi and mycotoxins in silage: An overview. J. Appl. Microbiol. 2013, 115, 637–643. [Google Scholar] [CrossRef] [PubMed]

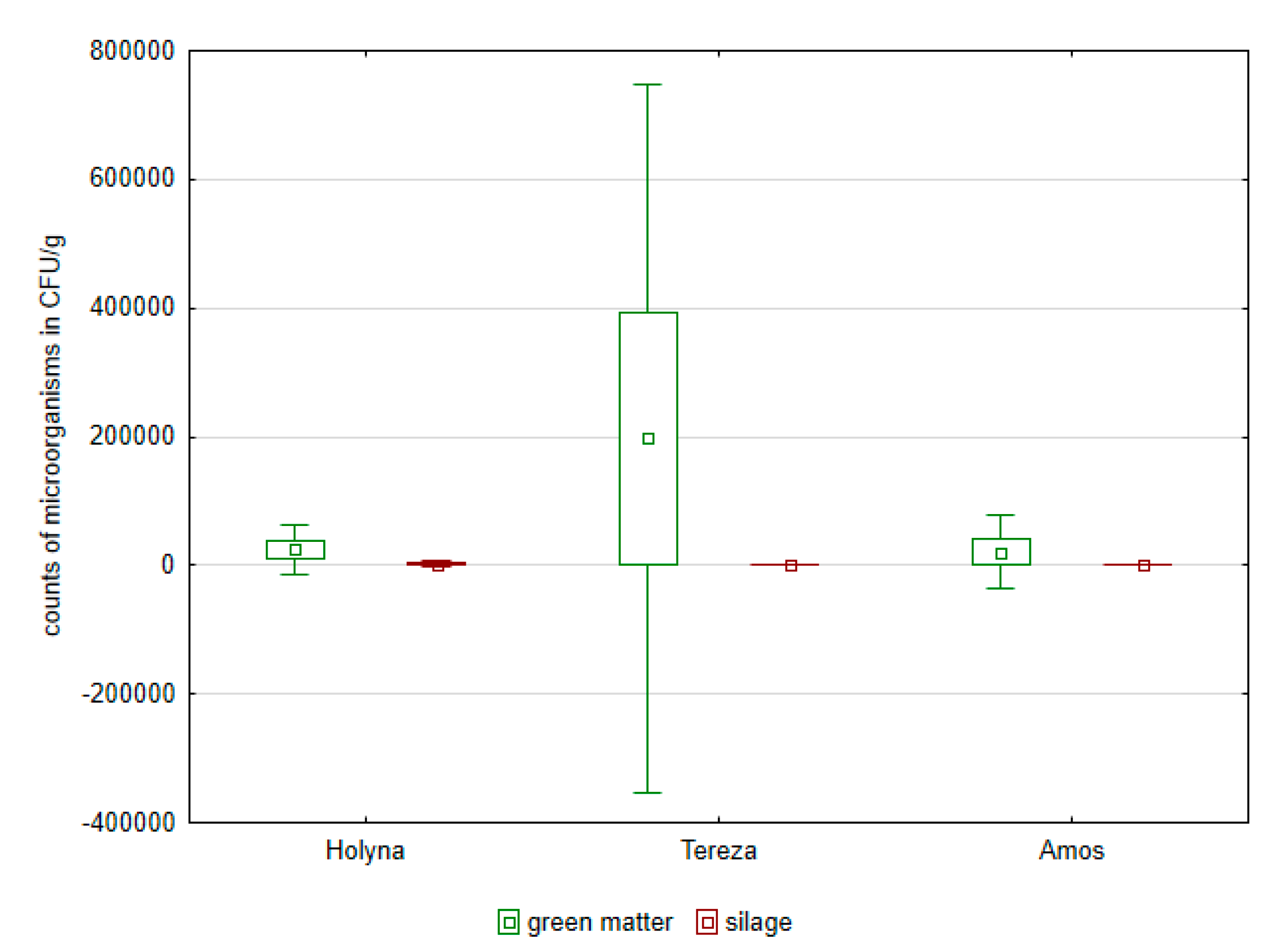
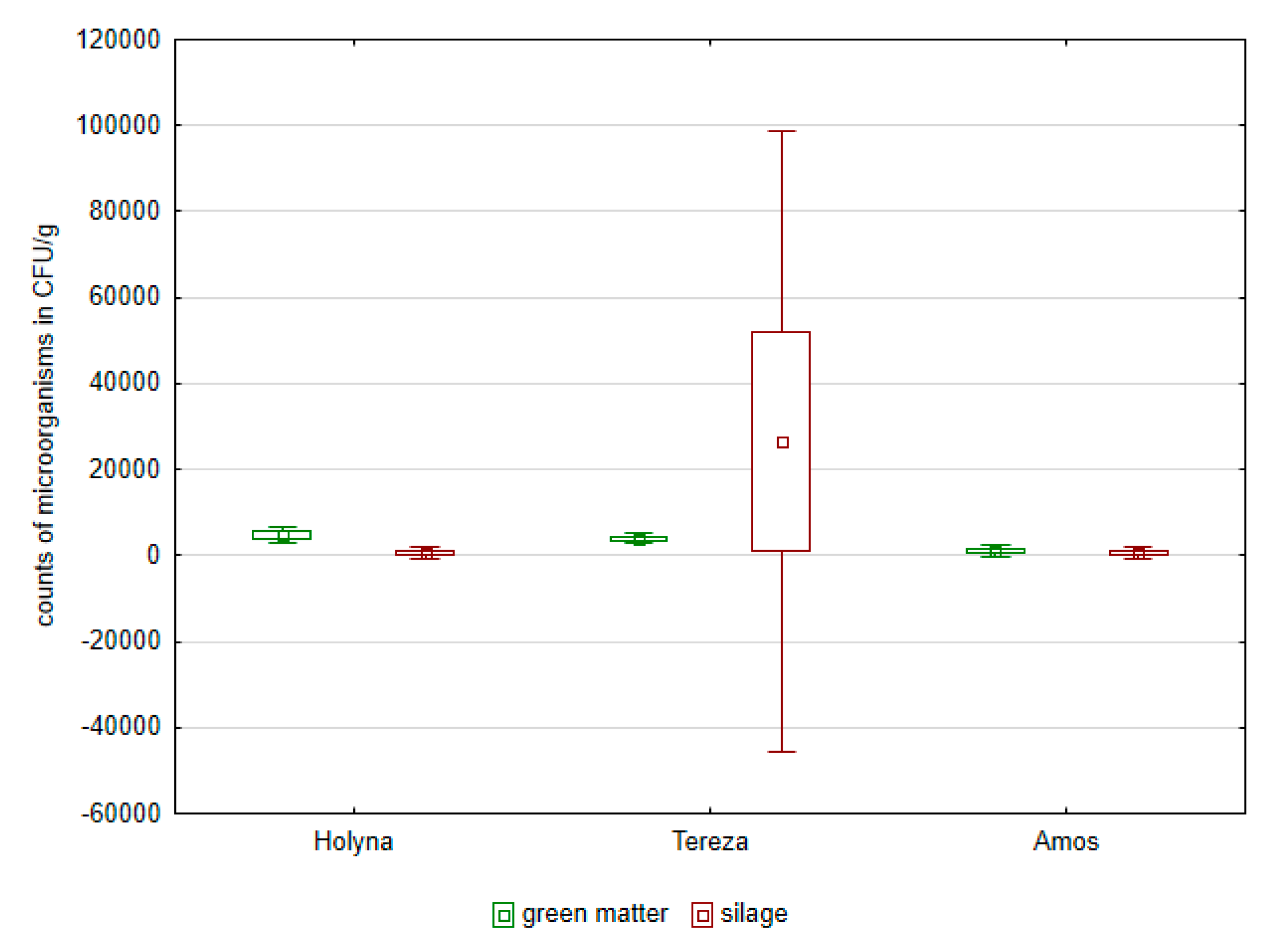

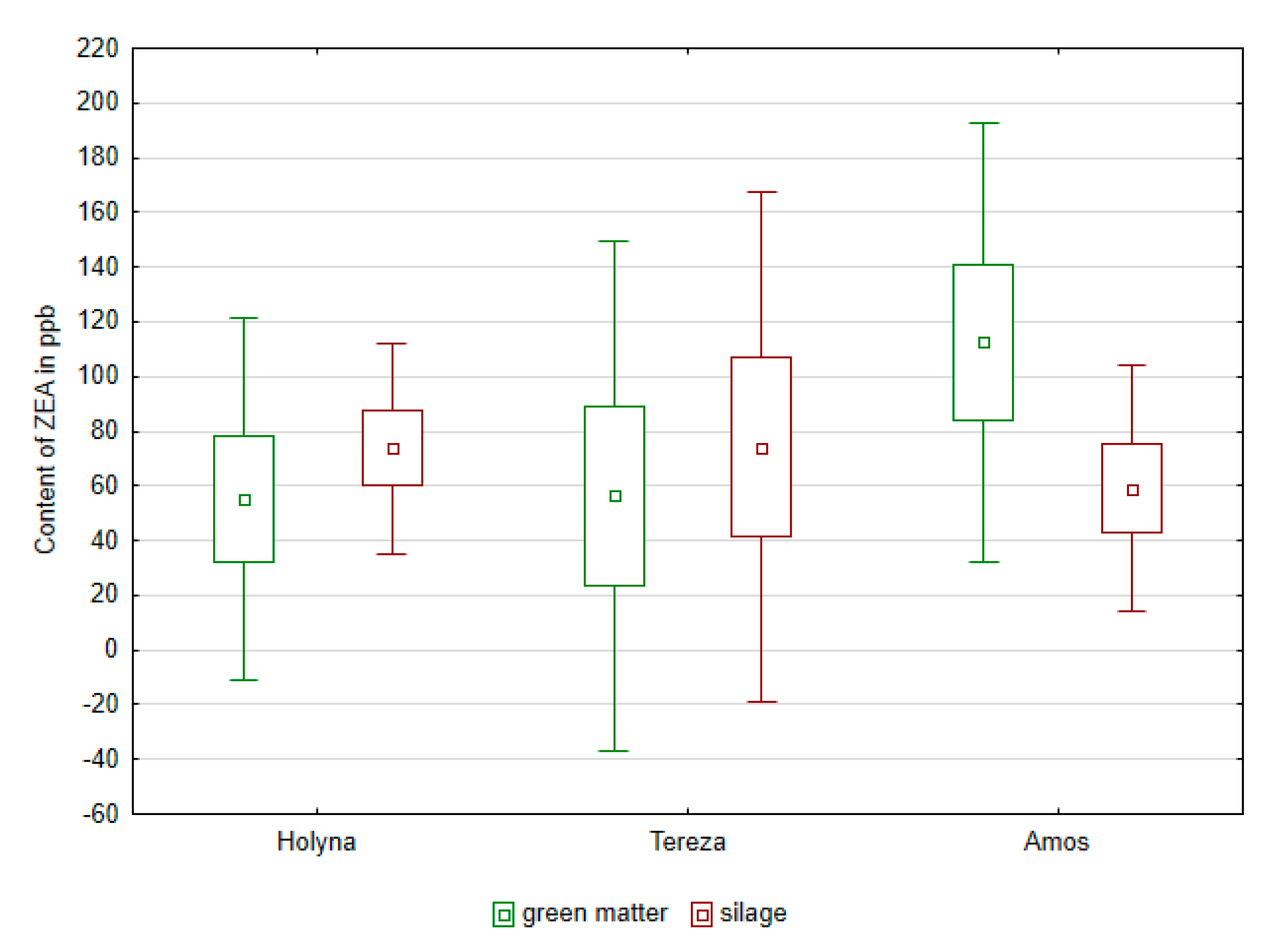

| Species | TNM | Lac Bacteria | Enterobacteria | Enterococcus | Fungi | Yeasts |
|---|---|---|---|---|---|---|
| Medicago sativa (Holyna) | 6.625 × 107 | 1.315 × 105 | 2.500 × 104 | 4.795 × 103 a | 5.250 × 105 | 5.500 × 103 |
| Medicago sativa (Tereza) | 1002.515 × 107 | 3.930 × 105 | 19.725 × 104 | 4.070 × 103 a,b | 8.822 × 105 | 9.500 × 103 |
| Trifolium pratense (Amos) | 3.834 × 107 | 0.525 × 105 | 2.100 × 104 | 1.000 × 103 b | 2.760 × 105 | 2.275 × 103 |
| p value | 0.4649 | 0.4430 | 0.5306 | 0.0330 | 0.7412 | 0.3482 |
| Factor | TNM | Lac Bacteria | Enterobacteria | Enterococcus | Fungi | Yeasts |
|---|---|---|---|---|---|---|
| Species (S) | ||||||
| Medicago sativa (Holyna) | 9.783 × 109 | 6.027 × 108 | 1.127 × 103 | 1.714 × 104 | 1.863 × 103 | 4.037 × 104 |
| Medicago sativa (Tereza) | 16.336 × 109 | 11.804 × 108 | 0.562 × 103 | 2.152 × 104 | 0.354 × 103 | 0.0016 × 104 |
| Trifolium pratense (Amos) | 1.418 × 109 | 0.237 × 108 | 0.234 × 103 | 1.725 × 104 | 3.123 × 103 | 4.145 × 104 |
| p value | 0.5545 | 0.4495 | 0.4526 | 0.9386 | 0.1491 | 0.4746 |
| Additives (P) | ||||||
| Control | 22.096 × 109 | 16.063 × 108 | 0.422 × 103 | 0.509 × 104 a | 1663 | 5.886 × 104 |
| Biological additive | 5.211 × 109 | 1.829 × 108 | 1.395 × 103 | 5.015 × 104 b | 2938 | 2.041 × 104 |
| Chemical additive | 0.231 × 109 | 0.176 × 108 | 0.106 × 103 | 0.673 × 104 a | 741 | 0.257 × 104 |
| p value | 0.2602 | 0.1722 | 0.1832 | 0.0023 | 0.2907 | 0.3356 |
| S × P | 0.5136 | 0.5935 | 0.9747 | 0.9915 | 0.6926 | 0.7375 |
| Factor | pH | Lac | Acet | PA | BA | Ethanol |
|---|---|---|---|---|---|---|
| Species (S) | ||||||
| Medicago sativa (Holyna) | 4.64 a | 7.33 a | 3.43 a | 0.032 | 1.056 a,b | 0.995 a |
| Medicago sativa (Tereza) | 4.81 a | 6.25 a | 3.52 a | 0.058 | 1.279 a | 1.275 b |
| Trifolium pratense (Amos) | 4.14 b | 11.25 b | 2.59 b | 0.000 | 0.000 b | 1.193 a,b |
| p value | 0.0000 | 0.0000 | 0.0041 | 0.0731 | 0.0205 | 0.0301 |
| Additives (P) | ||||||
| Control | 4.82 a | 9.03 | 3.59 a | 0.000 a | 1.056 a,b | 1.555 a |
| Biological additive | 4.54 a | 7.99 | 4.07 a | 0.091 b | 1.279 a | 1.350 a |
| Chemical additive | 4.22 b | 7.81 | 1.88 b | 0.000 a | 0.000 b | 0.557 b |
| p value | 0.0000 | 0.4344 | 0.0000 | 0.0004 | 0.0003 | 0.0000 |
| S × P | 0.0002 | 0.4679 | 0.0001 | 0.0369 | 0.0292 | 0.0465 |
| Factor | Him | Tym | Put | Cad | Spd | Spm | BA |
|---|---|---|---|---|---|---|---|
| Species (S) | |||||||
| Medicago sativa (Holyna) | 4.5 | 233.5 | 241.0 | 21.7 | 2.50 | 219.5 | 722.7 |
| Medicago sativa (Tereza) | 16.5 | 459.8 | 211.0 | 71.5 | 1.83 | 204.8 | 965.8 |
| Trifolium pratense (Amos) | 9.0 | 73.0 | 356.8 | 50.8 | 3.00 | 225.5 | 872.2 |
| p value | 0.2873 | 0.1513 | 0.7087 | 0.3857 | 0.6857 | 0.8686 | 0.8045 |
| Additives (P) | |||||||
| Control | 7.5 | 321.8 | 410.5 | 80.2 | 2.83 | 197.7 | 1174.8 |
| Biological additive | 12.5 | 420.3 | 282.7 | 63.7 | 3.00 | 225.8 | 1008.2 |
| Chemical additive | 9.0 | 24.2 | 115.7 | 0.17 | 1.50 | 226.3 | 377.7 |
| p value | 0.7882 | 0.1251 | 0.3145 | 0.0992 | 0.4885 | 0.7200 | 0.1269 |
| S × P | 0.5617 | 0.6775 | 0.4641 | 0.7238 | 0.4930 | 0.5826 | 0.5572 |
| Factor | DON | ZEA |
|---|---|---|
| Species (S) | ||
| Medicago sativa (Holyna) | 0.4517 | 115.35 |
| Medicago sativa (Tereza) | 0.3883 | 125.08 |
| Trifolium pratense (Amos) | 0.4983 | 115.55 |
| p value | 0.7198 | 0.7861 |
| Additives (P) | ||
| Control | 0.7417 a | 76.66 a |
| Biological additive | 0.2933 b | 144.04 b |
| Chemical additive | 0.3033 b | 137.28 b |
| p value | 0.0129 | 0.0031 |
| S × P | 0.9620 | 0.0953 |
| BA | DON | ZEA |
|---|---|---|
| Him | −0.14 | 0.30 |
| Tym | −0.17 | −0.29 |
| Put | 0.61 | 0.12 |
| Cad | 0.18 | −0.25 |
| Spd | 0.18 | 0.13 |
| Spm | −0.12 | −0.06 |
| BA | 0.43 | −0.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skladanka, J.; Adam, V.; Zitka, O.; Mlejnkova, V.; Kalhotka, L.; Horky, P.; Konecna, K.; Hodulikova, L.; Knotova, D.; Balabanova, M.; et al. Comparison of Biogenic Amines and Mycotoxins in Alfalfa and Red Clover Fodder Depending on Additives. Int. J. Environ. Res. Public Health 2017, 14, 418. https://doi.org/10.3390/ijerph14040418
Skladanka J, Adam V, Zitka O, Mlejnkova V, Kalhotka L, Horky P, Konecna K, Hodulikova L, Knotova D, Balabanova M, et al. Comparison of Biogenic Amines and Mycotoxins in Alfalfa and Red Clover Fodder Depending on Additives. International Journal of Environmental Research and Public Health. 2017; 14(4):418. https://doi.org/10.3390/ijerph14040418
Chicago/Turabian StyleSkladanka, Jiri, Vojtech Adam, Ondrej Zitka, Veronika Mlejnkova, Libor Kalhotka, Pavel Horky, Klara Konecna, Lucia Hodulikova, Daniela Knotova, Marie Balabanova, and et al. 2017. "Comparison of Biogenic Amines and Mycotoxins in Alfalfa and Red Clover Fodder Depending on Additives" International Journal of Environmental Research and Public Health 14, no. 4: 418. https://doi.org/10.3390/ijerph14040418
APA StyleSkladanka, J., Adam, V., Zitka, O., Mlejnkova, V., Kalhotka, L., Horky, P., Konecna, K., Hodulikova, L., Knotova, D., Balabanova, M., Slama, P., & Skarpa, P. (2017). Comparison of Biogenic Amines and Mycotoxins in Alfalfa and Red Clover Fodder Depending on Additives. International Journal of Environmental Research and Public Health, 14(4), 418. https://doi.org/10.3390/ijerph14040418










