Prevalence and Correlates of Suspected Nonalcoholic Fatty Liver Disease in Chinese Children
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NAFLD | nonalcoholic fatty liver disease |
| CHNS | China Health and Nutrition Surveys |
| WC | waist circumference |
| BP | blood pressure |
| ALT | serum alanine aminotransferase |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| Hb | hemoglobin |
| UA | serum uric acid |
| TC | total cholesterol |
| HDL | high-density lipoprotein cholesterol |
| LDL | low-density lipoprotein cholesterol |
| TG | triglyceride |
| OR | odds ratio |
| CI | confidence interval |
References
- Adams, L.A.; Angulo, P.; Lindor, K.D. Nonalcoholic fatty liver disease. Can. Med. Assoc. J. 2005, 172, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Erickson, S.K. Nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, S412–S416. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, W.; Socha, P. Non-alcoholic fatty liver disease in children. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, D.; Rousso, I.; Mavromichalis, I. Update on non-alcoholic fatty liver disease in children. Clin. Nutr. 2007, 26, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Bottazzo, G.; DeVito, R.; Marcellini, M.; Mingrone, G.; Nobili, V. Nonalcoholic fatty liver disease in children. J. Am. Coll. Nutr. 2008, 27, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.J.; Klingensmith, G.; Zeitler, P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Longnecker, M.P.; Lawlor, D. Prevalence of elevated alanine aminotransferase among us adolescents and associated factors: NHANES 1999–2004. Gastroenterology 2007, 133, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.S.; Barlow, S.E.; Dietz, W.H. Prevelance of abnormal serum aminotransferase values in overweight and obese adolescents. J. Pediatr. 2000, 136, 727–733. [Google Scholar] [CrossRef]
- Alisi, A.; Locatelli, M.; Nobili, V. Nonalcoholic fatty liver disease in children. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Patton, H.M.; Sirlin, C.; Behling, C.; Middleton, M.; Schwimmer, J.B.; Lavine, J.E. Pediatric nonalcoholic fatty liver disease: A critical appraisal of current data and implications for future research. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Mencin, A.A.; Lavine, J.E. Nonalcoholic fatty liver disease in children. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Del Col, A.; Agosti, F.; Mazzilli, G.; Bellentani, S.; Tiribelli, C.; Bedogni, G. Predictors of non-alcoholic fatty liver disease in obese children. Eur. J. Clin. Nutr. 2007, 61, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.; Lawlor, D.A.; Fraser, A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Wan, Y.-P.; Zhang, S.-J.; Lu, L.-P.; Chen, Z.-Q.; Liu, H.; Jiang, X.-M.; Luo, K.-L.; Cai, W. Nonalcoholic fatty liver disease prevalence in urban school-aged children and adolescents from the yangtze river delta region: A cross-sectional study. Asia Pac. J. Clin. Nutr. 2015, 24, 281–288. [Google Scholar] [PubMed]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort profile: The china health and nutrition survey—Monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Malespin, M.; Sleesman, B.; Lau, A.; Wong, S.S.; Cotler, S.J. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese American children. J. Clin. Gastroenterol. 2015, 49, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.; Du, S.; Popkin, B.M. The China health and nutrition survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; He, D.; Hu, Y.; Zhou, D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: The china health and nutrition survey in 2009. Prev. Med. 2013, 57, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, F.; Li, X.; Wu, L.; Zhang, Y.; Cheng, Y.; Zhou, W.; Huang, G. Blood pressure percentiles by age and height for non-overweight Chinese children and adolescents: Analysis of the China health and nutrition surveys 1991–2009. BMC Pediatr. 2013, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, C.; Zong, X.; Zhang, Y. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Chin. J. Pediatr. 2009, 47, 493–498. (In Chinese) [Google Scholar]
- Song, P.; Li, X.; Gasevic, D.; Flores, A.B.; Yu, Z. BMI, waist circumference reference values for Chinese school-aged children and adolescents. Int. J. Environ. Res. Public Health 2016, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Falkner, B.; Daniels, S.R. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension 2004, 44, 387–388. [Google Scholar] [CrossRef] [PubMed]
- WHO. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Available online: http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf (accessed on 2 March 2017).
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Gröber-Grätz, D.; Widhalm, K.; De Zwaan, M.; Reinehr, T.; Blüher, S.; Schwab, K.O.; Wiegand, S.; Holl, R.W. Body mass index or waist circumference: Which is the better predictor for hypertension and dyslipidemia in overweight/obese children and adolescents? Association of cardiovascular risk related to body mass index or waist circumference. Horm. Res. Pediatr. 2013, 80, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.; Alisi, A.; Saccari, A.; De Vito, R.; Vania, A.; Nobili, V. Nonalcoholic fatty liver in children and adolescents: An overview. J. Adolesc. Health 2012, 51, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Alkhouri, N.; Carter-Kent, C.; Monti, L.; Devito, R.; Lopez, R.; Feldstein, A.E.; Nobili, V. Ultrasonographic quantitative estimation of hepatic steatosis in children with nonalcoholic fatty liver disease (NAFLD). J. Pediatr. Gastroenterol. Nutr. 2011, 53, 190. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013, 162, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, F.; Türkkan, D.; Yüksel, I.; Kara, S.; Celik, N.; Şamdancı, E. Fatty liver disease in an autopsy series of children and adolescents. Hippokratia 2012, 16, 61. [Google Scholar] [PubMed]
- Giorgio, V.; Prono, F.; Graziano, F.; Nobili, V. Pediatric nonalcoholic fatty liver disease: Old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Manco, M.; Vania, A.; Nobili, V. Pediatric nonalcoholic fatty liver disease in 2009. J. Pediatr. 2009, 155, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; McGreal, N.; Deutsch, R.; Finegold, M.J.; Lavine, J.E. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005, 115, e561–e565. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2007, 65, S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; Ling, S.; Roberts, E.A. Anthropometric and metabolic characteristics in children with clinically diagnosed nonalcoholic fatty liver disease. Pediatr. Child Health 2008, 13, 111. [Google Scholar]
- Yap, J.Y.; O‘Connor, C.; Mager, D.R.; Taylor, G.; Roberts, E.A. Diagnostic challenges of nonalcoholic fatty liver disease (NAFLD) in children of normal weight. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Carter-Kent, C.; Elias, M.; Feldstein, A.E. Atherogenic dyslipidemia and cardiovascular risk in children with nonalcoholic fatty liver disease. Clin. Lipidol. 2011, 6, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jia, J. Update on epidemiology of hepatitis B and C in China. J. Gastroenterol. Hepatol. 2013, 28, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Smith, M.K.; Wang, L.; Su, Y.; Wang, L.; Guo, W.; Wang, L.; Cui, Y.; Wang, N. Hepatitis C virus infection in China: An emerging public health issue. J. Viral Hepat. 2015, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: Position paper of the espghan hepatology committee. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 700–713. [Google Scholar] [CrossRef] [PubMed]
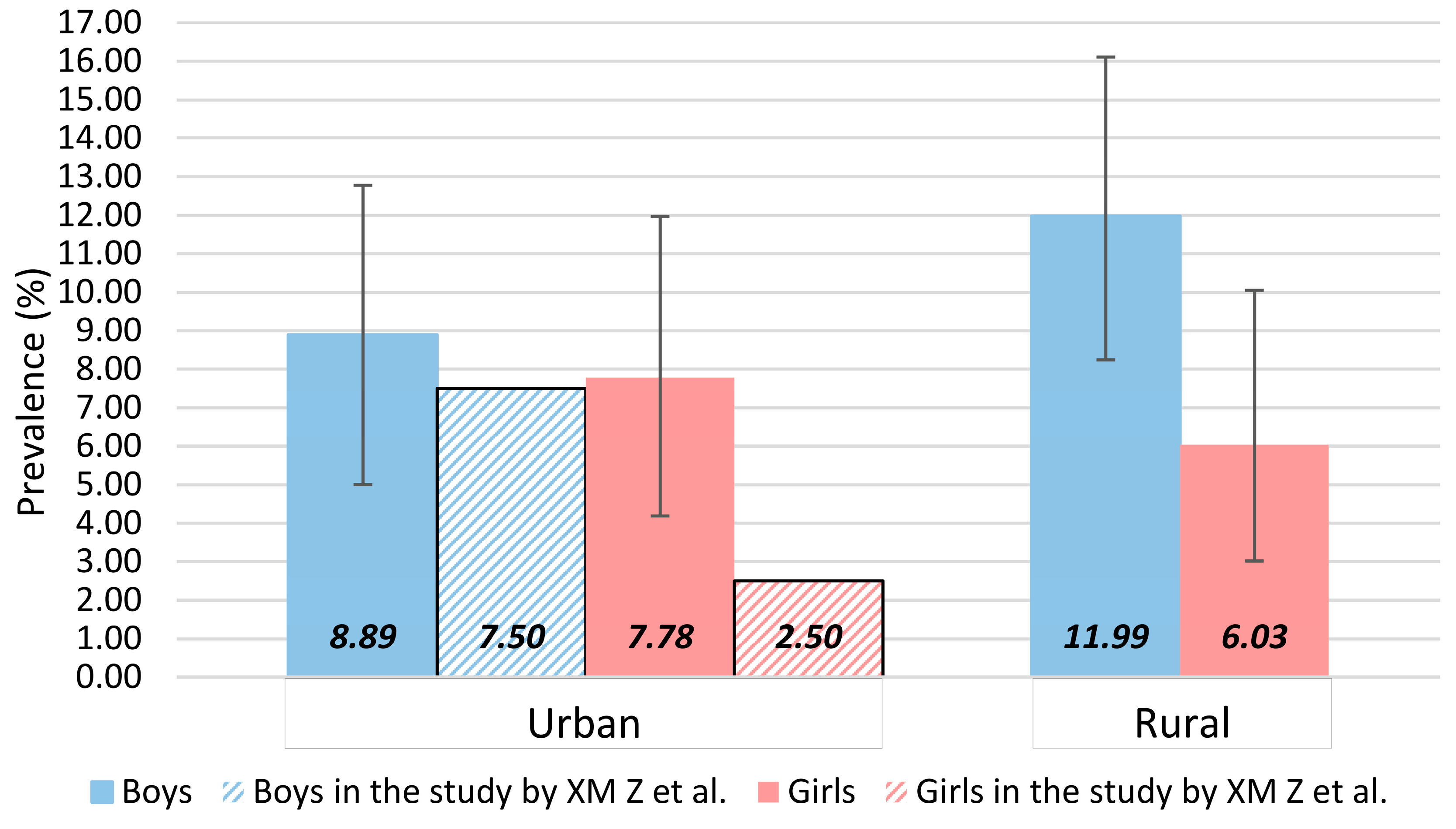
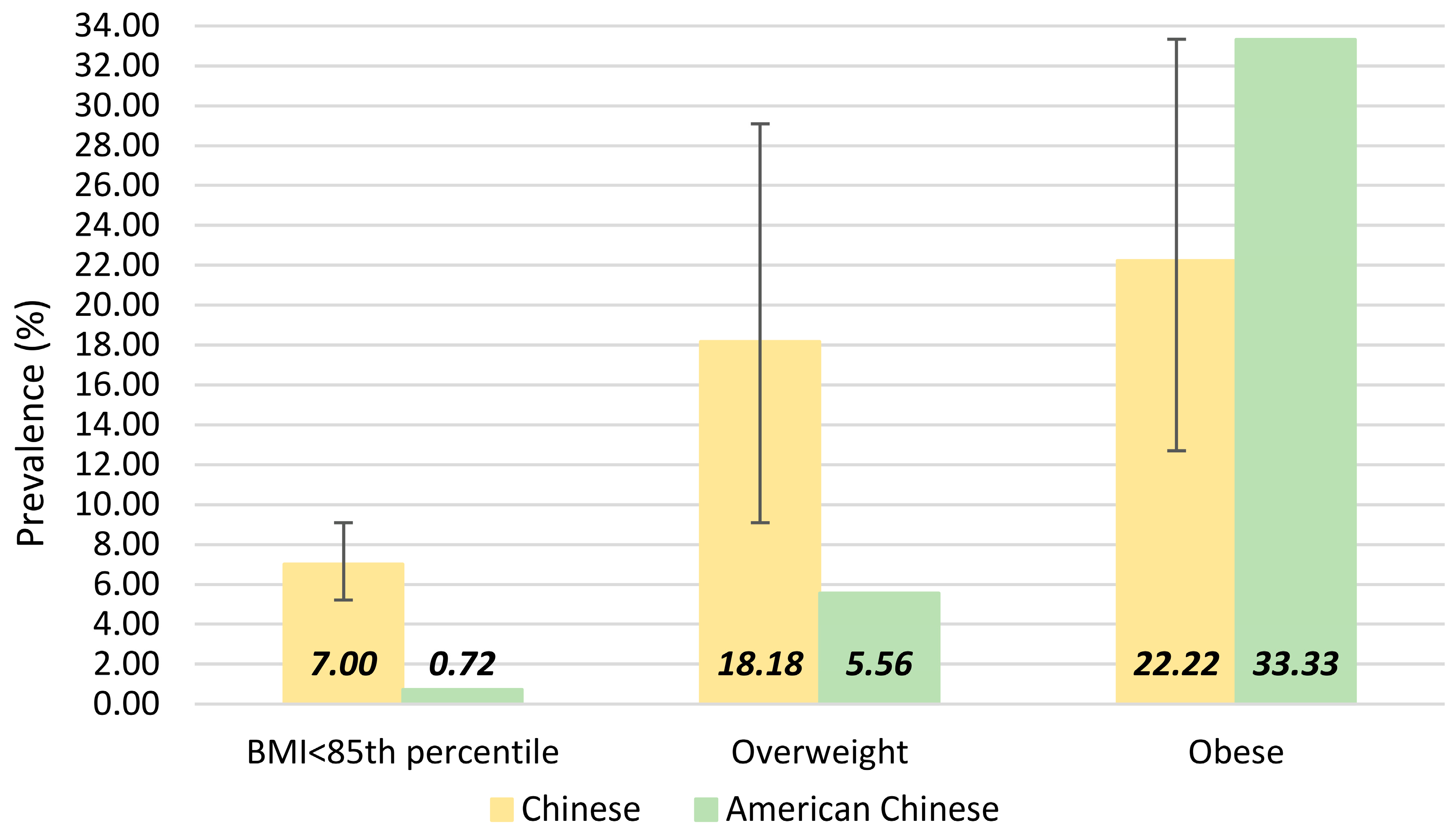
| Characteristic | Total (n = 831) | Boys (n = 456) | Girls (n = 375) | Urban (n = 347) | Rural (n = 466) |
|---|---|---|---|---|---|
| Age (years) | 12.39 ± 3.05 | 12.34 ± 3.07 | 12.45 ± 3.02 | 12.72 ± 3.02 | 11.91 ± 2.84 $ |
| Weight (kg) | 39.64 ± 13.13 | 40.29 ± 13.92 | 38.86 ± 12.08 | 42.45 ± 13.28 | 37.56 ± 12.63 $ |
| Height (cm) | 147.30 ± 15.85 | 148.24 ± 17.05 | 146.19 ± 14.24 | 150.63 ± 15.69 | 144.84 ± 15.54 $ |
| BMI (kg/m2) | 17.77 ± 3.33 | 17.80 ± 3.38 | 17.72 ± 3.26 | 18.24 ± 3.33 | 17.42 ± 3.29 $ |
| WC (cm) | 63.26 ± 9.61 | 64.09 ± 10.15 | 62.27 ± 8.82 * | 64.43 ± 9.14 | 62.41 ± 9.86 $ |
| SBP (mmHg) | 100.04 ± 13.02 | 100.48 ± 13.41 | 99.51 ± 12.54 | 100.76 ± 11.93 | 99.51 ± 13.76 |
| DBP (mmHg) | 66.67 ± 9.50 | 66.81 ± 9.48 | 66.50 ± 9.53 | 67.19 ± 9.39 | 66.28 ± 9.57 |
| Hb (g/L) | 137.65 ± 16.54 | 140.45 ± 16.31 | 134.26 ± 16.20 * | 137.07 ± 15.64 | 137.94 ± 16.77 |
| UA (μmol/L) | 310.14 ± 84.99 | 328.74 ± 89.5 | 287.51 ± 73.14 * | 314.12 ± 87.03 | 307.2 ± 84.24 |
| TC (mmol/L) | 3.88 ± 0.70 | 3.81 ± 0.70 | 3.96 ± 0.69 * | 3.93 ± 0.71 | 3.84 ± 0.69 |
| HDL (mmol/L) | 1.44 ± 0.53 | 1.42 ± 0.35 | 1.46 ± 0.70 | 1.48 ± 0.72 | 1.41 ± 0.34 |
| LDL (mmol/L) | 2.21 ± 0.88 | 2.18 ± 1.05 | 2.24 ± 0.62 | 2.23 ± 0.93 | 2.18 ± 0.85 |
| TG (mmol/L) | 1.01 ± 0.72 | 0.98 ± 0.74 | 1.04 ± 0.68 | 1.02 ± 0.76 | 0.99 ± 0.69 |
| ALT (IU/L) | 13.00 (10.00–17.00) | 14.00 (11.00–18.00) | 12.00 (9.00–15.00) * | 12.00 (9.00–17.00) | 13.00 (10.00–17.00) $ |
| Characteristic | Suspected NAFLD (n = 75) | Without Suspected NAFLD (n = 756) | p-Value |
|---|---|---|---|
| Age (years) | 12.85 ± 3.01 | 12.34 ± 3.05 | 0.173 |
| Gender | |||
| Boys (%) | 49 (10.75%) | 407 (89.25%) | 0.056 |
| Girls (%) | 26 (6.93%) | 349 (93.07%) | |
| Residence | |||
| Urban (%) | 29 (8.36%) | 318 (91.64%) | 0.593 |
| Rural (%) | 44 (9.44%) | 422 (90.56%) | |
| Weight (kg) | 44.96 ± 14.96 | 39.12 ± 12.83 | <0.001 * |
| Height (cm) | 149.24 ± 15.27 | 147.11 ± 15.91 | 0.282 |
| BMI (kg/m2) | 19.74 ± 4.61 | 17.57 ± 3.11 | <0.001 * |
| WC (cm) | 69.93 ± 11.68 | 62.61 ± 9.13 | <0.001 * |
| SBP (mmHg) | 103.95 ± 12.50 | 99.66 ± 13.02 | 0.008 * |
| DBP (mmHg) | 69.54 ± 8.76 | 66.39 ± 9.53 | 0.008 * |
| Hb (g/L) | 141.15 ± 19.90 | 137.30 ± 16.14 | 0.109 |
| UA (μmol/L) | 347.12 ± 86.86 | 306.47 ± 83.98 | <0.001 * |
| TC (mmol/L) | 4.20 ± 1.01 | 3.85 ± 0.65 | 0.004 * |
| HDL (mmol/L) | 1.32 ± 0.36 | 1.45 ± 0.55 | 0.039 * |
| LDL (mmol/L) | 2.43 ± 0.82 | 2.18 ± 0.88 | 0.021 * |
| TG (mmol/L) | 1.49 ± 1.23 | 0.96 ± 0.63 | <0.001 * |
| ALT (IU/L) | 32.00 (27.00–50.00) | 12.00 (10.00–15.00) | <0.001 * |
| Characteristic | Adjusted OR (95% CI) | p-Value |
|---|---|---|
| BMI category | ||
| <85th percentile r | 1.00 | |
| Overweight and obesity | 2.02 (1.04–3.93) | 0.038 |
| Abdominal obesity | ||
| No r | 1.00 | |
| Yes | 2.76 (1.35–5.66) | 0.006 |
| Hyperuricemia a | ||
| No r | 1.00 | |
| Yes | 2.31 (1.38–3.89) | 0.002 |
| Elevated TC | ||
| No r | 1.00 | |
| Yes | 4.70 (2.04–10.79) | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, P.; Yu, J.; Wang, M.; Chang, X.; Wang, J.; An, L. Prevalence and Correlates of Suspected Nonalcoholic Fatty Liver Disease in Chinese Children. Int. J. Environ. Res. Public Health 2017, 14, 465. https://doi.org/10.3390/ijerph14050465
Song P, Yu J, Wang M, Chang X, Wang J, An L. Prevalence and Correlates of Suspected Nonalcoholic Fatty Liver Disease in Chinese Children. International Journal of Environmental Research and Public Health. 2017; 14(5):465. https://doi.org/10.3390/ijerph14050465
Chicago/Turabian StyleSong, Peige, Jinyue Yu, Manli Wang, Xinlei Chang, Jiawen Wang, and Lin An. 2017. "Prevalence and Correlates of Suspected Nonalcoholic Fatty Liver Disease in Chinese Children" International Journal of Environmental Research and Public Health 14, no. 5: 465. https://doi.org/10.3390/ijerph14050465
APA StyleSong, P., Yu, J., Wang, M., Chang, X., Wang, J., & An, L. (2017). Prevalence and Correlates of Suspected Nonalcoholic Fatty Liver Disease in Chinese Children. International Journal of Environmental Research and Public Health, 14(5), 465. https://doi.org/10.3390/ijerph14050465





