Influence of Nano-Hydroxyapatite on the Metal Bioavailability, Plant Metal Accumulation and Root Exudates of Ryegrass for Phytoremediation in Lead-Polluted Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Pot Experiment
2.2. Pb Content Determination
2.3. Determination of pH
2.4. Organic Acids Analysis
2.5. Community Bureau of Reference Sequential Extraction Tests
2.6. Statistical Analysis
3. Results and Discussion
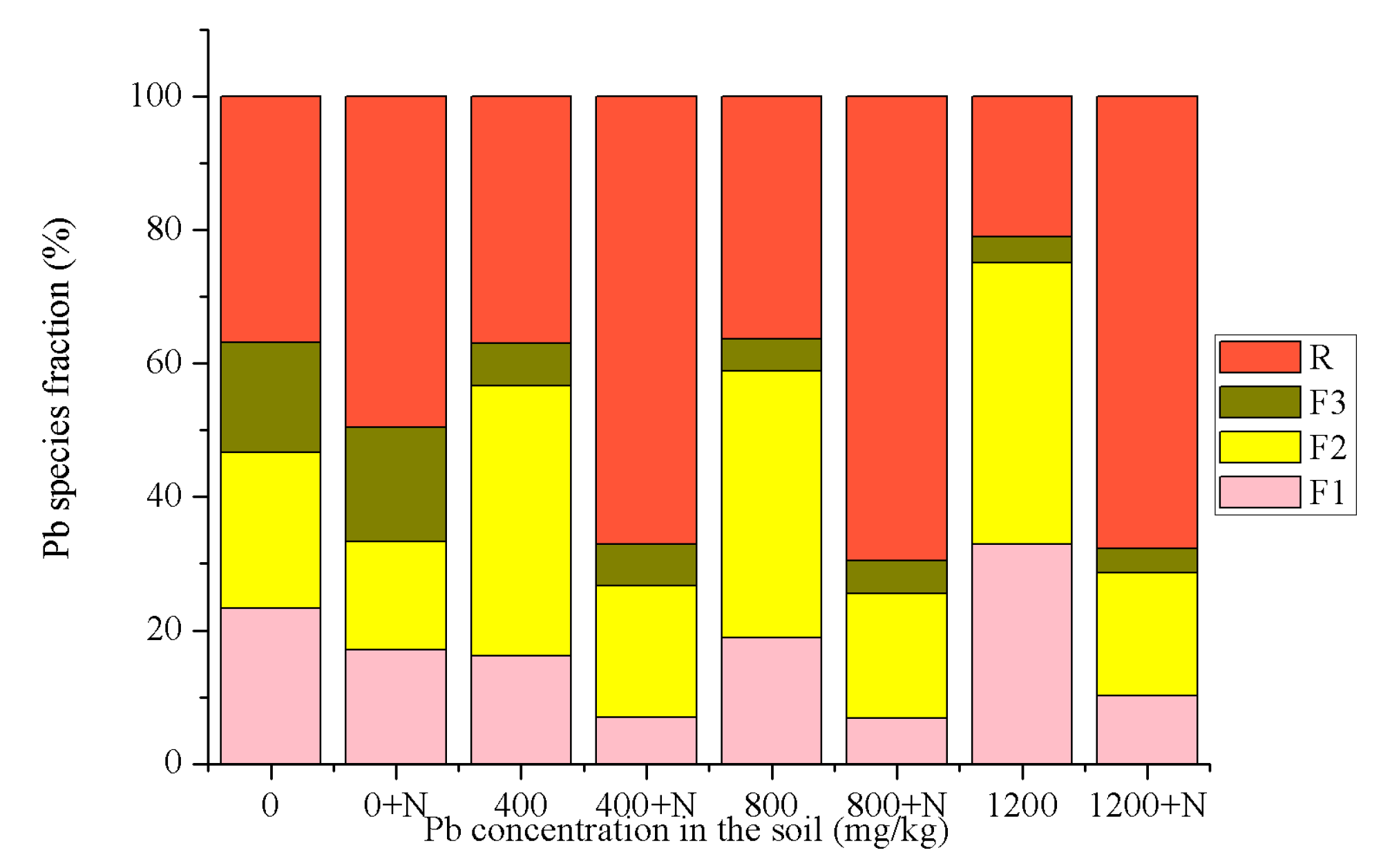
3.1. Speciation Analysis of Pb in Soil
3.2. Effect of Nano-Hydroxyapatite on the pH of Soil
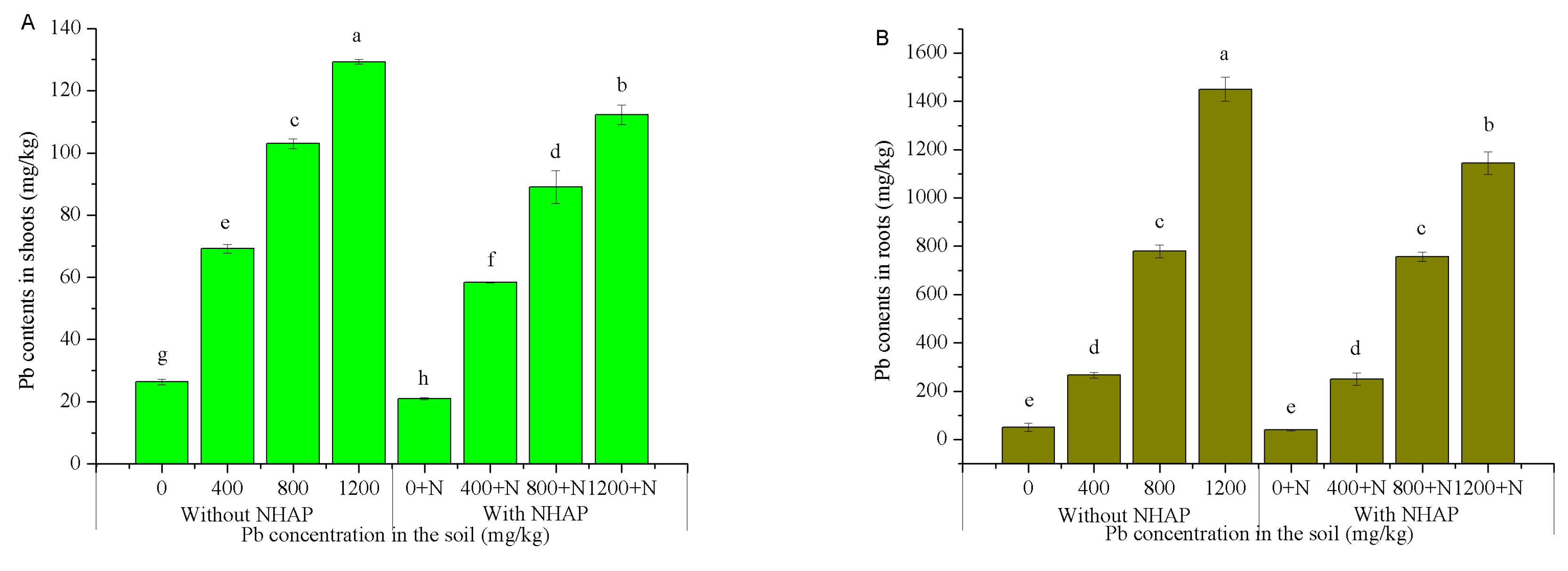
3.3. Effect of Nano-Hydroxyapatite on Pb Accumulation in Ryegrass
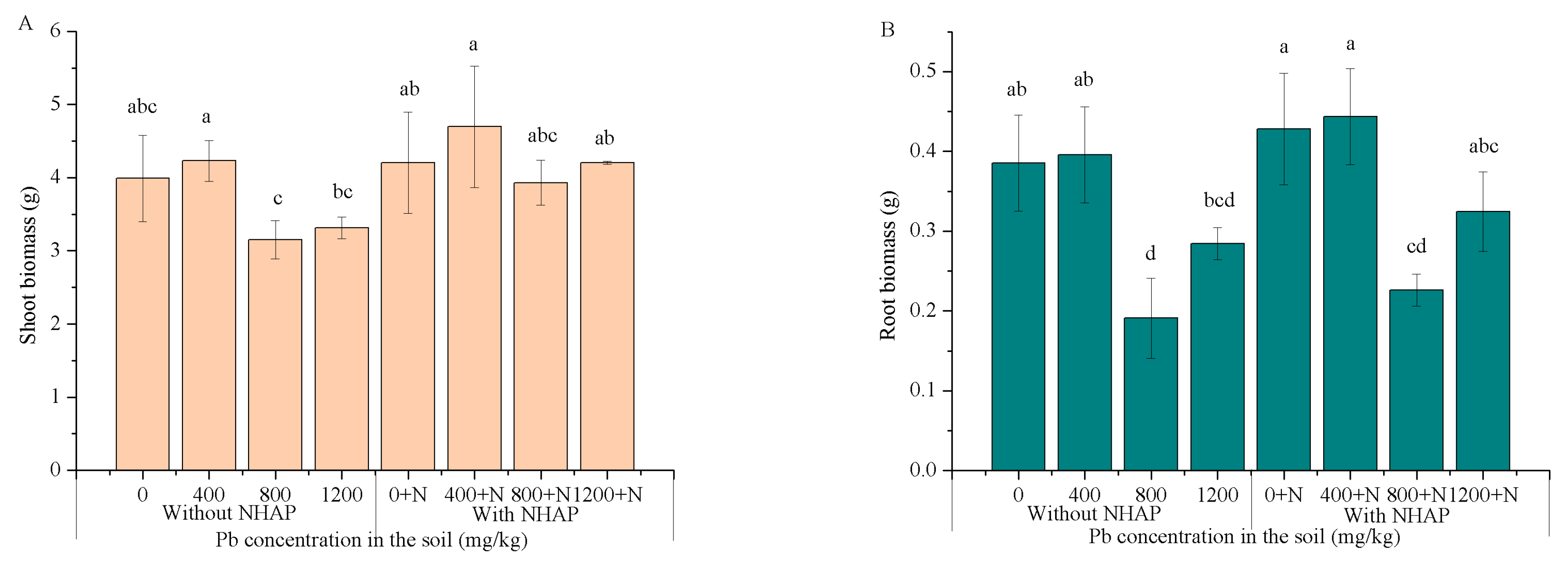
3.4. Plant Growth and Biomass
3.5. Organic Acid Response to Nano-Hydroxyapatitein the Ryegrass Rhizosphere
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shrivastava, R.; Upreti, R.K.; Chaturvedi, U.C. Various cells of the immune system and intestine differ in their capacity to reduce hexavalent chromium. FEMS Immunol. Med. Microbiol. 2003, 38, 65–70. [Google Scholar] [CrossRef]
- Miller, G.; Begonia, G.; Begonia, M.; Ntoni, J. Bioavailability and uptake of lead by coffeeweed (Sesbania exaltata Raf.). Int. J. Environ. Res. Public Health 2008, 5, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Boisson, J.; Ruttens, A.; Mench, M.; Vangronsveld, J. Evaluation of hydroxyapatite as a metal immobilizing soil additive for the remediation of polluted soils. Part 1. Influence of hydroxyapatite on metal exchangeability in soil, plant growth and plant metal accumulation. Environ. Pollut. 1999, 104, 225–233. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A.A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M. Lead biotransformation potential of allochthonous Bacillus sp. SKK11 with sesame oil cake extract in mine soil. RSC Adv. 2015, 5, 54564–54570. [Google Scholar]
- Chinese Environmental Protection Administration. Environmental Quality Standards for Soils; GB15618-1995; Chinese Environmental Protection Administration: Beijing, China.
- Guo, G.; Zhou, Q.; Ma, L.Q. Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: A review. Environ. Monit. Assess. 2006, 116, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Sanghera, G.S.; Athokpam, H. Phytoremediation: Curing soil problems with crops. Afr. J. Agric. Res. 2012, 7, 3991–4002. [Google Scholar]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D.; Grubb, D.G. Phosphate application to firing range soils for Pb immobilization: The unclear role of phosphate. J. Hazard. Mater. 2007, 144, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.Z.; Liu, N.N.; Zhu, L.Y. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2009, 158, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- Gu, C.; Bai, Y.; Tao, T. Effect of sewage sludge amendment on heavy metal uptake and yield of ryegrass seedling in a mudflat soil. J. Environ. Qual. 2013, 42, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, W.; Li, X.L.; Shen, S.G.; Liang, S.X.; Liu, C.Q.; Shan, L.Y. Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol. Eng. 2016, 95, 25–29. [Google Scholar] [CrossRef]
- Fang, X.; You, M.P.; Barbetti, M.J. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. Eur. J. Plant Pathol. 2012, 134, 619–629. [Google Scholar] [CrossRef]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 169–234. [Google Scholar] [CrossRef]
- Chacón, N.; Herrera, I.; Flores, S.; González, J.A.; Nassar, J.M. Chemical, physical, and biochemical soil properties and plant roots as affected by native and exotic plants in neotropical arid zones. Biol. Fertil. Soils 2009, 45, 321–328. [Google Scholar] [CrossRef]
- Gillman, G.P.; Bruce, R.C.; Davey, B.G. A comparison of methods used for determination of cation exchange capacity. Commun. Soil Sci. Plant Anal. 1983, 14, 1005–1014. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Mcgrouther, K. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.V.; Nascimento, C.W.; Silva, W.M. Citric acid-assisted phytoextraction of lead in the field: The use of soil amendments. Water Air Soil Pollut. 2014, 225, 1–9. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Yılmaz, V.; Kartal, Ş. An assessment on metal sources by multivariate analysis and speciation of metals in soil samples using the BCR sequential extraction procedure. Clean-Soil Air Water 2010, 38, 713–718. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Ryan, J.A.; Zhang, P.; Hesterberg, D. Formation of Chloropyromorphite in a Lead-Contaminated Soil Amended with Hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.C.; Bitton, G. Correlation between heavy metal toxicity and metal fractions of contaminated soils in Korea. Bull. Environ. Contam. Toxicol. 2003, 70, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hashimoto, Y.; Moon, D.H.; Lee, S.S.; Ok, Y.S. Immobilization of lead in a Korean military shooting range soil using eggshell waste: An integrated mechanistic approach. J. Hazard. Mater. 2012, 4, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fang, Z.; Zheng, L. Remediation of lead contaminated soil by biochar-supported nano-hydroxyapatite. Ecotoxicol. Environ. Saf. 2016, 132, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, M.; Lin, W. The research of nanoparticle and microparticle hydroxyapatite amendment in multiple heavy metals contaminated soil remediation. J. Nanomater. 2014, 17, 1–8. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Menabue, L.; Saladini, M. Removal of cadmium ion by means of synthetic hydroxyapatite. Waste Manag. 2002, 22, 853–857. [Google Scholar] [CrossRef]
- Silber, A.; Yones, L.B.; Dori, I. Rhizosphere pH as a result of nitrogen levels and NH4/NO3 ratio and its effect on zinc availability and on growth of rice flower (Ozothamnus diosmifolius). Plant Soil 2004, 262, 205–213. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Ding, Y.Z.; Song, Z.G.; Feng, R.W.; Guo, J.K. Interaction of organic acids and pH on multi-heavy metal extraction from alkaline and acid mine soils. Int. J. Environ. Sci. Technol. 2014, 11, 1–10. [Google Scholar] [CrossRef]
- Debela, F.; Arocena, J.M.; Thring, R.W.; Whitcombe, T. Organic acid-induced release of lead from pyromorphite and its relevance to reclamation of Pb-contaminated soils. Chemosphere 2010, 80, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 85, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Liu, H.L.; Liu, W.; Zuo, Q.Q. Effect of low-molecular-weight organic acids on nano-hydroxyapatite adsorption of cadmium and lead. J. Biomater. Tissue Eng. 2016, 6, 433–439. [Google Scholar] [CrossRef]

| Treatment | Pb-Spiked Content (mg/kg) | Addition Amount of NHAP (g) |
|---|---|---|
| 0 mg/kg | 0 | 0 |
| 0 mg/kg + NHAP | 0 | 1.5 |
| 400 mg/kg | 400 | 0 |
| 400 mg/kg + NHAP | 400 | 1.5 |
| 800 mg/kg | 800 | 0 |
| 800 mg/kg + NHAP | 800 | 1.5 |
| 1200 mg/kg | 1200 | 0 |
| 1200 mg/kg + NHAP | 1200 | 1.5 |
| Fraction | Reagent | Shaking Time and Temperature |
|---|---|---|
| Exchangeable (F1) | 40 mL of 0.11 mol/L CH3COOH | 16 h at 25 °C |
| Reducible (iron/manganese oxyhydroxides) (F2) | 40 mL of 0.5 mol/L NH2OH·HCl | 16 h at 25 °C |
| Oxidizable (organic matter and sulfides) (F3) | 10 mL of 8.8 mol/L H2O2, twice, cool and add 50 mL of 1 mol/L NH4Ac | 1 h at 25 °C, 1 h at 85 °C, 1 h at 85 °C, 16 h at 25 °C |
| Residual (R) | HNO3-H2O2-HF | Microwave digestion |
| Exogenous Pb Concentration (mg/kg) | The Rhizosphere Soil pH | |
|---|---|---|
| Without NHAP | With NHAP | |
| 0 | 8.64 ± 0.10 a,b | 8.66 ± 0.03 a |
| 400 | 8.69 ± 0.04 a | 8.73 ± 0.02 a |
| 800 | 8.51 ± 0.20 b | 8.64 ± 0.03 a,b |
| 1200 | 8.12 ± 0.01 d | 8.20 ± 0.01 c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Li, J.; Liu, W.; Zuo, Q.; Liang, S.-x. Influence of Nano-Hydroxyapatite on the Metal Bioavailability, Plant Metal Accumulation and Root Exudates of Ryegrass for Phytoremediation in Lead-Polluted Soil. Int. J. Environ. Res. Public Health 2017, 14, 532. https://doi.org/10.3390/ijerph14050532
Ding L, Li J, Liu W, Zuo Q, Liang S-x. Influence of Nano-Hydroxyapatite on the Metal Bioavailability, Plant Metal Accumulation and Root Exudates of Ryegrass for Phytoremediation in Lead-Polluted Soil. International Journal of Environmental Research and Public Health. 2017; 14(5):532. https://doi.org/10.3390/ijerph14050532
Chicago/Turabian StyleDing, Ling, Jianbing Li, Wei Liu, Qingqing Zuo, and Shu-xuan Liang. 2017. "Influence of Nano-Hydroxyapatite on the Metal Bioavailability, Plant Metal Accumulation and Root Exudates of Ryegrass for Phytoremediation in Lead-Polluted Soil" International Journal of Environmental Research and Public Health 14, no. 5: 532. https://doi.org/10.3390/ijerph14050532





