Changes in Urinary Phthalate Metabolite Levels Before and After the Phthalate Contamination Event and Identification of Exposure Sources in a Cohort of Taiwanese Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Information Collection
2.2. Questionnaire on Environmental Exposures
2.3. Urine Sample Preparation and Measurement
2.4. Statistical Analysis
3. Results
3.1. Basic Demographics of the Study Population
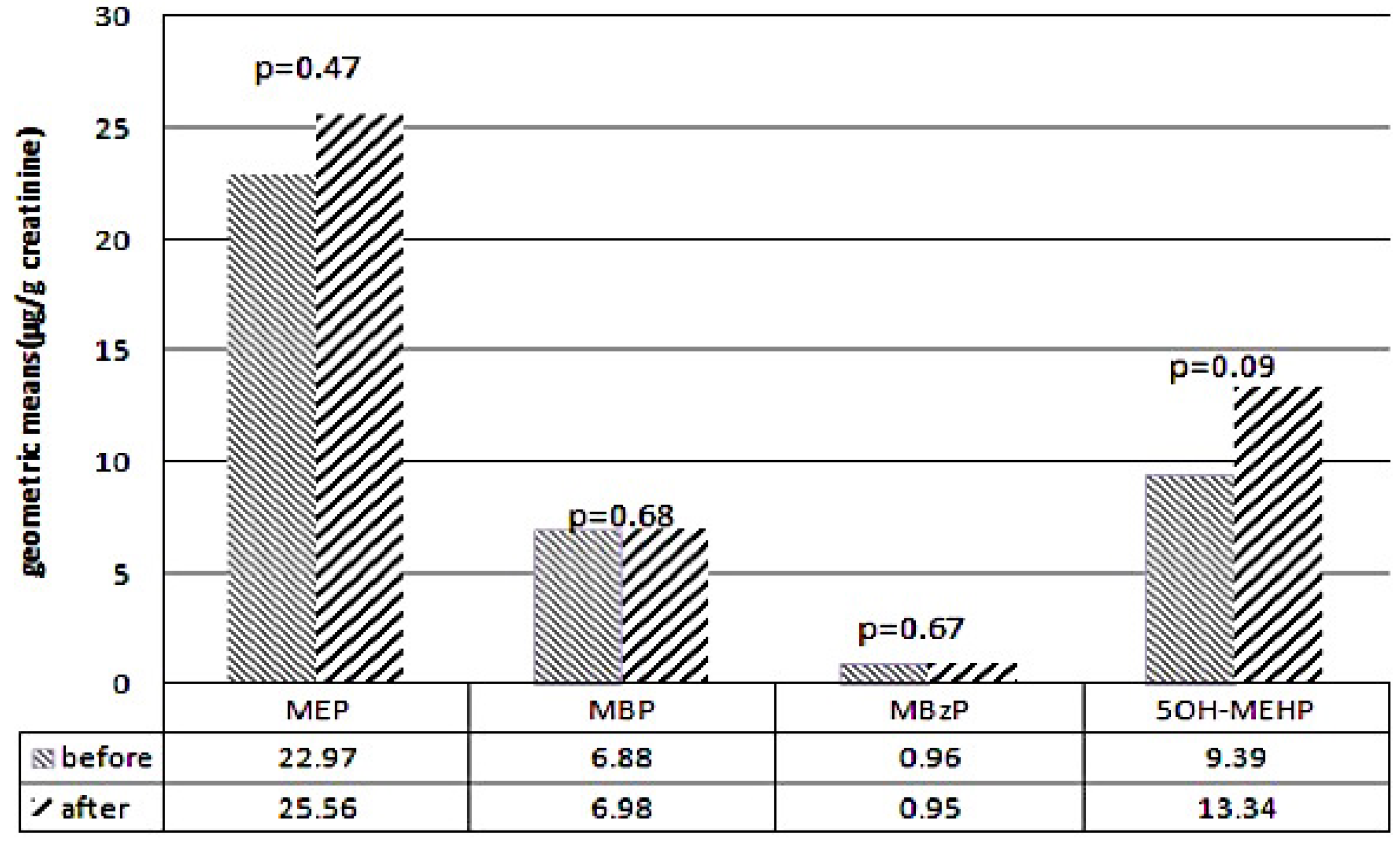
3.2. Comparison of Metabolite Levels Before and After the DEHP Food Contamination Event
3.3. Environmental Risk Factors for Higher Urinary Phthalate Metabolite Levels
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DEP | Diethylphthalate |
| DBP | Dibutyl phthalate |
| BBzP | Butylbenzyl phthalate |
| DEHP | di(2-Ethylhexyl) phthalate |
| MEP | Monoethyl phthalate |
| MBP | Monobutyl phthalate |
| MBzP | Monobenzyl phthalate |
| 5OH-MEHP | Mono(2-ethyl-5-hydroxyhexyl)phthalate |
| UPLC-MS/MS | Ultra-performance liquid chromatography coupled with tandem mass spectrometry |
References
- Wu, M.T.; Wu, C.F.; Wu, J.R.; Chen, B.H.; Chen, E.K.; Chao, M.C.; Liu, C.K.; Ho, C.K. The public health threat of phthalate-tainted foodstuffs in Taiwan: The policies the government implemented and the lessons we learned. Environ. Int. 2012, 44, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hauser, R.; Goldman, R.H. Taiwan food scandal: The illegal use of phthalates as a clouding agent and their contribution to maternal exposure. Food Chem. Toxicol. 2013, 58, 362−368. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.; Hochner-Celnikier, D.; Calafat, A.M.; Needham, L.L.; Amitai, Y.; Wormser, U.; Richter, E. Phthalate exposure among pregnant women in Jerusalem, Israel: Results of a pilot study. Environ. Int. 2009, 35, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC Fourth National Report on Human Exposure to Environmental Chemicals. Available online: http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 15 May 2012).
- Wang, I.J.; Lin, C.C.; Lin, Y.J.; Hsieh, W.S.; Chen, P.C. Early life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ. Int. 2014, 62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profiles for di(2-ethylhexyl)phthalate(DEHP); Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2002.
- Chen, F.P.; Chien, M.H. Lower concentrations of phthalates induce proliferation in human breast cancer cells. Climacteric 2014, 17, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, S.D.; Lei, X.; Ling, Y.S.; Luo, Y.; Liu, Q. Association of PAEs with Precocious Puberty in Children: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2015, 12, 15254–15268. [Google Scholar] [CrossRef] [PubMed]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, B.; Lind, P.M.; Lind, L. Serum levels of monobenzyl phthalate (MBzP) is related to carotid atherosclerosis in the elderly. Environ. Res. 2014, 133, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Montes, L.P.; Hernandez-Valero, M.A.; Flores-Pimentel, D.; García-Fábila, M.; Amaya-Chávez, A.; Barr, D.B.; Borja-Aburto, V.H. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J. Dev. Orig. Health Dis. 2013, 4, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Toshima, H.; Suzuki, Y.; Imai, K.; Yoshinaga, J.; Shiraishi, H.; Mizumoto, Y.; Hatakeyama, S.; Onohara, C.; Tokuoka, S. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: A pilot study on exposure and semen quality. Int. J. Hyg. Environ. Health 2012, 215, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Tranfo, G.; Caporossi, L.; Paci, E.; Aragona, C.; Romanzi, D.; De Carolis, C.; De Rosa, M.; Capanna, S.; Papaleo, B.; Pera, A. Urinary phthalate monoesters concentration in couples with infertility problems. Toxicol. Lett. 2012, 213, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bornehag, C.G.; Nanberg, E. Phthalate exposure and asthma in children. Int. J. Androl. 2010, 33, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Karmaus, W.J.J. The effect of phthalate exposure and filaggrin gene variants on atopic dermatitis. Environ. Res. 2015, 136, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Davis, K.; Boylan, K.; Fisher, M.; Fu, J. Processed data for CHMS 2007–2009: Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6–19 years of age. Data Brief 2016, 8, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Hong, Y.C.; Shin, C.H.; Lee, Y.A.; Lim, Y.H.; Kim, B.N. The effects of maternal and children phthalate exposure on the neurocognitive function of 6-year-old children. Environ. Res. 2017, 156, 519–525. [Google Scholar] [CrossRef] [PubMed]
- TFDA. FDA Joint Investigation Found Food Additive tainted with DEHP (Plasticizer) Manufactured by Yu Shen Company; Taiwan Food and Drug Administration: Taipei, Taiwan, 2011.
- Silva, M.J.; Slakman, A.R.; Reidy, J.A.; Preau, J.L. Jr.; Herbert, A.R.; Samandar, E.; Needham, L.L.; Calafat, A.M. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J. Chromatogr. B 2004, 805, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cayman Chemical Company. Creatinine (Urinary) Colorimetric Assay Kit. Available online: https://www.caymanchem.com/pdfs/500701.pdf (accessed on 27 October 2016).
- Wu, C.F.; Chen, B.H.; Shiea, J.; Chen, E.K.; Liu, C.K.; Chao, M.C.; Ho, C.K.; Wu, J.R.; Wu, M.T. Temporal changes of urinary oxidative metabolites of di(2-ethylhexyl)phthalate after the 2011 phthalate incident in Taiwanese children: findings of a six month follow-up. Environ. Sci. Technol. 2013, 47, 13754–13762. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Sullivan, K.D.; Dhungana, B. Phthalate and non-phthalate plasticizers in indoor dust from childcare facilities, salons, and homes across the USA. Environ. Pollut. 2017, 230, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014. [CrossRef] [PubMed]
- Sioen, I.; Fierens, T.; Van Holderbeke, M.; Geerts, L.; Bellemans, M.; De Maeyer, M.; Servaes, K.; Vanermen, G.; Boon, P.E.; De Henauw, S. Phthalates dietary exposure and food sources for Belgian preschool children and adults. Environ. Int. 2012, 48, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castro, M.I.; Olea-Serrano, M.F.; Rivas-Velasco, A.M.; Medina-Rivero, E.; Ordonez-Acevedo, L.G.; De Leon-Rodriguez, A. Phthalates and bisphenols migration in Mexican food cans and plastic food containers. Bull. Environ. Contam. Toxicol. 2011, 86, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; Andre, L.C.; Cardeal, Z.L. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int. J. Environ. Res. Public Health 2014, 11, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Chen, J.S.; Tang, C.L.; Mao, I.F. The internal exposure of Taiwanese to phthalate-an evidence of intensive use of plastic materials. Environ. Int. 2008, 4, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Meeker, J.D.; Peterson, K.E.; Lee, J.M.; Pace, G.G.; Cantoral, A.; Téllez-Rojo, M.M. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 2013, 93, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Kuo, P.L.; Guo, Y.L.; Liao, P.C.; Lee, C.C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Factor-Litvak, P.; Insel, B.; Calafat, A.M.; Liu, X.; Perera, F.; Rauh, V.A.; Whyatt, R.M. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PLoS ONE 2014, 9, e114003. [Google Scholar] [CrossRef]
- Swan, S.H.; Sathyanarayana, S.; Barrett, E.S.; Janssen, S.; Liu, F.; Nguyen, R.H.; Redmon, J.B.; TIDES Study Team. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 2015, 30, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, F.; Jönsson, B.; Bornehag, C.G. PVC flooring is related to human uptake of phthalates in infants. Indoor Air 2013, 23, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Bekö, G.; Callesen, M.; Weschler, C.J.; Toftum, J.; Langer, S.; Sigsgaard, T.; Høst, A.; Kold Jensen, T.; Clausen, G. Phthalate exposure through different pathways and allergic sensitization in preschool children with asthma, allergic rhinoconjunctivitis and atopic dermatitis. Environ. Res. 2015, 137, 432–439. [Google Scholar]
- Kelley, K.E.; Hernandez-Diaz, S.; Chaplin, E.L.; Hauser, R.; Mitchell, A.A. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect. 2012, 120, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, N.Y.; Lee, C.C.; Wang, J.Y.; Li, Y.C.; Chang, H.W.; Chen, C.Y.; Bornehag, C.G.; Wu, P.C.; Sundell, J.; Su, H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerESIV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Bolt, H.M.; Angerer, J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 2004, 78, 123–130. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | % | GM (s.e.) MEP (µg/g Creatinine) | GM (s.e.) MBP (µg/g Creatinine) | GM (s.e.) MBzP (µg/g Creatinine) | GM (s.e.) 5OH-MEHP (µg/g Creatinine) | |
|---|---|---|---|---|---|---|
| Mother | ||||||
| Maternal age | <34 years | 82.2 | 21.98 (3.71) | 5.99 (3.32) | 0.95 (2.92) | 9.03 (3.16) |
| ≥34 years | 17.8 | 23.57 (4.31) | 6.89 (3.06) | 0.96 (3.22) | 9.39 (2.77) | |
| Maternal education | <College | 69.2 | 21.98 (3.94) | 6.11 (3.22) | 0.94 (2.97) | 8.58 (3.25) |
| ≥College | 30.8 | 21.33 (3.49) | 6.05 (3.35) | 0.94 (2.89) | 10.28 (2.86) | |
| Maternal nationality | Taiwan | 93.8 | 22.20 (3.86) | 5.99 (3.22) * | 0.94 (2.94) | 9.21 (3.16) |
| others | 6.2 | 16.78 (2.59) | 8.67 (4.10) | 0.95 (3.03) | 7.24 (2.83) | |
| Maternal history of atopy | No | 64.9 | 23.10 (3.97) | 6.17 (3.46) | 0.86 (2.92) | 9.49 (3.13) |
| Yes | 35.1 | 20.49 (3.60) | 6.11 (3.06) | 1.16 (3.00) | 8.25 (3.06) | |
| Children | ||||||
| Gender | Male | 57.7 | 23.81 (3.94) | 7.69 (3.90) | 0.93 (2.97) | 9.97 (3.29) |
| Female | 42.3 | 21.98 (3.86) | 5.93 (3.42) | 0.99 (2.89) | 8.76 (3.46) | |
| Birth weight | <2500 gm | 5.4 | 15.33 (3.97) | 7.46 (2.97) | 1.02 (2.72) | 7.03 (3.03) |
| ≥2500 gm | 94.6 | 22.87 (3.74) | 6.17 (3.32) | 0.92 (2.94) | 9.58 (3.22) | |
| Gestational age | <37 weeks | 9.0 | 22.20 (3.29) | 6.05 (2.94) | 0.70 (2.32) | 9.12 (2.64) |
| ≥37 weeks | 91.0 | 21.98 (3.82) | 6.30 (3.35) | 0.97 (2.97) | 9.30 (3.25) | |
| Parity | <2 | 82.5 | 22.65 (3.63) | 6.49 (3.35) * | 0.96 (2.86) * | 9.30 (3.19) |
| ≥2 | 17.5 | 19.30 (4.53) | 5.00 (2.97) | 0.83 (3.25) | 8.25 (2.89) | |
| Breast feeding | No | 23.6 | 17.81 (3.71) | 5.93 (3.60) | 0.90 (3.25) | 8.58 (3.16) |
| Yes | 76.4 | 22.65 (3.71) | 6.36 (3.25) | 0.94 (2.86) | 9.39 (3.22) | |
| Family income per year | ||||||
| <600,000 NT dollars | 31.3 | 21.12 (3.53) | 6.55 (3.22) | 0.91 (2.86) | 9.49 (3.19) | |
| 600,000–1,500,000 NT dollars | 60.7 | 23.81 (3.94) | 6.42 (3.39) | 0.96 (3.06) | 9.97 (3.29) | |
| >1,500,000 NT dollars | 8.0 | 20.49 (4.31) | 6.05 (3.39) | 0.99 (3.39) | 6.69 (2.72) | |
| Environmental Factors | % | MEP (µg/g Creatinine) | MBP (µg/g Creatinine) | MBzP (µg/g Creatinine) | 5OH-MEHP (µg/g Creatinine) | |
|---|---|---|---|---|---|---|
| Hot food with plastic bags | Seldom | 20.5 | 21.67 (4.07) | 6.54 (3.00) | 0.96 (3.04) | 7.66 (2.73) |
| Usually | 79.5 | 25.02 (3.95) | 7.15 (3.36) | 0.93 (2.82) | 11.08 (3.43) | |
| p value | 0.83 | 0.40 | 0.88 | 0.15 | ||
| PVC film stored or heated food | Seldom | 42.4 | 19.82 (3.70) | 7.24 (3.19) | 0.83 (2.73) | 9.59 (3.04) |
| Usually | 57.6 | 27.82 (4.13) | 6.86 (3.35) | 1.01 (2.92) | 10.46(3.43) | |
| p value | 0.22 | 0.73 | 0.37 | 0.33 | ||
| Microwave heated food | Seldom | 41.6 | 21.06 (3.81) | 6.08 (2.81) | 0.97 (2.67) | 9.34 (2.82) |
| Usually | 58.4 | 26.58 (4.07) | 7.78 (3.58) | 0.90 (2.97) | 10.85 (3.64) | |
| p value | 0.29 | 0.02 * | 0.51 | 0.06 | ||
| Plastic bottle drinks | Seldom | 9.1 | 13.72 (2.74) | 6.10 (2.59) | 1.08 (3.58) | 9.68 (4.58) |
| Usually | 90.9 | 25.53 (4.05) | 7.12 (3.34) | 0.92 (2.77) | 10.25 (3.19) | |
| p value | <0.01 * | 0.06 | 0.87 | 0.51 | ||
| Plastic nursing bottle, nipple | Seldom | 82.4 | 23.16 (4.54) | 7.03 (3.22) | 0.93 (2.65) | 9.88 (3.32) |
| Usually | 17.6 | 25.13 (3.87) | 7.18 (3.76) | 1.06 (3.85) | 12.08 (3.48) | |
| p value | 0.58 | 0.76 | 0.28 | 0.77 | ||
| Products containing fragrance | Seldom | 97.9 | 24.15 (3.83) | 7.06 (3.29) | 0.94 (2.81) | 8.72 (2.20) |
| Usually | 2.1 | 80.07 (12.25) | 7.16 (4.51) | 1.44 (5.19) | 10.27 (3.38) | |
| p value | 0.02 * | 0.68 | 0.51 | 0.65 | ||
| Wash hands before eating | <2 meals/day | 3.7 | 24.37 (3.90) | 7.05 (3.22) | 0.93 (2.86) | 10.14 (3.24) |
| ≥2 meals/day | 96.3 | 21.41 (6.28) | 6.34 (5.18) | 0.69 (1.60) | 9.13 (4.38) | |
| p value | 0.55 | 0.45 | 0.04 * | 0.76 | ||
| Instant noodles intake | Seldom | 33.4 | 22.32 (3.99) | 6.88 (3.11) | 0.88 (2.66) | 9.83 (3.25) |
| Usually | 66.6 | 25.09 (3.97) | 7.32 (3.61) | 1.04 (3.19) | 10.97 (3.41) | |
| p value | 0.61 | 0.18 | 0.26 | 0.33 | ||
| PVC flooring | No | 78.6 | 23.27 (4.14) | 6.94 (3.15) | 0.88 (2.91) | 10.24 (3.30) |
| Yes | 21.4 | 24.16 (3.07) | 7.53 (3.82) | 0.95 (2.86) | 10.54 (3.27) | |
| p value | 0.34 | 0.21 | 0.63 | 0.94 | ||
| Incense burning at home | <3 times/month | 47.3 | 24.16 (3.95) | 6.61 (3.47) | 0.91 (2.7) | 11.14 (3.49) |
| Usually | 52.7 | 22.91 (3.85) | 7.50 (3.11) | 0.95 (3.01) | 9.51 (3.11) | |
| p value | 0.93 | 0.65 | 0.29 | 0.79 | ||
| ETS exposure | No | 46.8 | 26.40 (4.14) | 6.78 (3.34) | 0.89 (2.85) | 9.68 (3.36) |
| Yes | 53.2 | 22.97 (3.87) | 7.49 (3.26) | 0.98 (2.91) | 10.98 (3.24) | |
| p value | 0.94 | 0.92 | 0.34 | 0.84 | ||
| Dehumidifier at home | No | 57.9 | 24.01 (3.77) | 7.36 (3.34) | 0.94 (2.76) | 10.65 (3.33) |
| Yes | 42.1 | 23.98 (4.09) | 6.82 (3.23) | 0.94 (3.05) | 9.69 (3.31) | |
| p value | 0.88 | 0.42 | 0.58 | 0.60 | ||
| DEHP Contaminated Food Intake | % | MEP (µg/g Creatinine) | MBP (µg/g Creatinine) | MBzP (µg/g Creatinine) | 5OH-MEHP (µg/g Creatinine) |
|---|---|---|---|---|---|
| None | 67.9 | 16.47 (4.24) | 4.47 (3.14) | 0.59 (2.54) | 7.57 (2.66) |
| Juice, jelly | 6.2 | 16.65 (2.46) | 4.63 (2.13) | 0.57 (1.86) | 7.61 (1.82) |
| Sport drink | 17.7 | 24.15(4.23) | 5.97 (3.36) | 0.81 (3.16) | 9.23 (1.08) |
| Probiotics, vitamin supplements | 8.1 | 21.44(2.51) | 6.48(2.69) | 0.90 (2.86) | 19.19 (4.08) * |
| p value | 0.77 | 0.48 | 0.55 | <0.001 |
| Environmental Factors | % | MEP (µg/g Creatinine) | MBP (µg/g Creatinine) | MBzP (µg/g Creatinine) | 5OH-MEHP (µg/g Creatinine) | |
|---|---|---|---|---|---|---|
| Hot food with plastic bags | Seldom | 18.0 | 24.80 (4.35) | 6.97 (4.70) | 0.91 (2.78) | 12.29 (3.64) |
| Usually | 82.0 | 25.49 (4.21) | 7.02 (4.02) | 1.01 (3.26) | 12.92 (3.57) | |
| p value | 0.91 | 0.56 | 0.42 | 0.76 | ||
| PVC film stored or heated food | Seldom | 43.5 | 24.59 (4.51) | 6.61 (3.86) | 0.90 (2.83) | 11.26 (3.63) |
| Usually | 56.5 | 25.98 (4.03) | 7.94 (4.48) | 0.96 (2.91) | 13.36 (3.61) | |
| p value | 0.82 | 0.33 | 0.90 | 0.32 | ||
| Microwave heated food | Seldom | 46.6 | 21.49 (3.79) | 6.96 (3.84) | 0.90 (2.70) | 11.16 (3.36) |
| Usually | 53.4 | 29.32 (4.57) | 7.34 (4.40) | 0.95 (3.06) | 13.59 (3.85) | |
| p value | 0.22 | 0.37 | 0.37 | 0.03 * | ||
| Plastic bottle drinks | Seldom | 10.6 | 24.82 (4.30) | 7.01 (4.17) | 0.92 (2.85) | 12.36 (3.61) |
| Usually | 89.4 | 30.46 (3.64) | 8.57 (3.83) | 0.99 (2.99) | 12.77 (3.84) | |
| p value | 0.63 | 0.88 | 0.96 | 0.85 | ||
| Plastic nursing bottle, nipple | Seldom | 85.6 | 22.26 (4.11) | 6.62 (4.22) | 0.89 (2.75) | 11.32 (3.57) |
| Usually | 14.4 | 58.97 (4.30) | 12.02 (3.62) | 1.14 (3.49) | 21.01 (4.10) | |
| p value | 0.12 | 0.54 | 0.29 | 0.18 | ||
| Products containing fragrance | Seldom | 95.4 | 23.78 (4.04) | 6.95 (4.20) | 0.71 (1.37) | 12.00 (3.60) |
| Usually | 4.6 | 120.14 (6.71) | 15.48 (3.19) | 0.93 (2.91) | 32.83 (3.79) | |
| p value | <0.01 * | 0.72 | 0.34 | 0.02 * | ||
| Wash hands before eating | <2 meals/day | 4.3 | 41.09 (10.14) | 7.20 (4.12) | 0.94 (2.90) | 32.05 (4.36) |
| ≥2 meals/day | 95.7 | 24.82 (4.03) | 6.34 (4.61) | 0.64 (1.83) | 12.06 (3.50) | |
| p value | <0.01 * | 0.80 | 0.35 | 0.01 * | ||
| Instant noodles intake | Seldom | 37.3 | 25.84 (3.87) | 6.21 (4.15) | 0.88 (2.83) | 10.98 (3.47) |
| Usually | 62.7 | 25.09 (4.45) | 7.79 (4.10) | 1.00 (2.92) | 13.33 (3.70) | |
| p value | 0.47 | 0.83 | 0.41 | 0.26 | ||
| PVC flooring | No | 78.2 | 24.54 (4.13) | 5.12 (3.45) | 0.74 (2.45) | 10.37 (3.74) |
| Yes | 21.8 | 26.84 (3.87) | 7.68 (4.31) | 0.99 (2.94) | 12.91 (3.55) | |
| p value | 0.84 | 0.03 * | 0.04 * | 0.50 | ||
| Incense burning at home | <3 times/month | 51.5 | 28.78 (3.57) | 7.61 (4.43) | 0.83 (2.76) | 12.03 (3.47) |
| Usually | 48.5 | 21.56 (4.57) | 6.46 (3.86) | 1.04 (2.91) | 12.60 (3.74) | |
| p value | 0.71 | 0.27 | 0.52 | 0.45 | ||
| ETS exposure | No | 52.8 | 27.40 (4.23) | 6.22 (3.88) | 1.02 (2.86) | 11.51 (3.57) |
| Yes | 47.2 | 24.79 (4.23) | 8.22 (4.30) | 0.81 (2.68) | 13.46 (3.70) | |
| p value | 0.90 | 0.27 | 0.15 | 0.88 | ||
| Dehumidifier at home | No | 55.2 | 29.53 (4.18) | 7.72 (4.29) | 0.97 (3.17) | 13.34 (3.61) |
| Yes | 44.8 | 19.89 (3.89) | 6.11 (3.84) | 0.90 (2.44) | 11.31 (3.46) | |
| p value | 0.30 | 0.32 | 0.09 | 0.45 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-F.; Wang, I.-J. Changes in Urinary Phthalate Metabolite Levels Before and After the Phthalate Contamination Event and Identification of Exposure Sources in a Cohort of Taiwanese Children. Int. J. Environ. Res. Public Health 2017, 14, 935. https://doi.org/10.3390/ijerph14080935
Huang C-F, Wang I-J. Changes in Urinary Phthalate Metabolite Levels Before and After the Phthalate Contamination Event and Identification of Exposure Sources in a Cohort of Taiwanese Children. International Journal of Environmental Research and Public Health. 2017; 14(8):935. https://doi.org/10.3390/ijerph14080935
Chicago/Turabian StyleHuang, Chian-Feng, and I-Jen Wang. 2017. "Changes in Urinary Phthalate Metabolite Levels Before and After the Phthalate Contamination Event and Identification of Exposure Sources in a Cohort of Taiwanese Children" International Journal of Environmental Research and Public Health 14, no. 8: 935. https://doi.org/10.3390/ijerph14080935






