1. Introduction
Diarrhoea is one of the major causes of child morbidity and mortality. For example, in 2015, it was estimated that more than half a million children under the age of five died from diarrhoeal diseases [
1]. Nearly half of these deaths occurred in sub-Saharan Africa. Although child mortality due to diarrhoeal diseases has declined annually by 6.5% since the establishment of the UN Millennium Development Goal 4 (MDG4) in 2000, in 2015, 9% of all child deaths were still due to diarrhoeal diseases, and morbidity from diarrhoea remains unacceptably high [
2]. Climate change impacts and will increasingly influence human health, and is expected to affect waterborne diseases, including diarrhoeal diseases [
3]. Environmental change is a major risk factor for public and global health, including children’s health [
4]. Children may experience greater risk of infectious diseases like diarrhoea due to rise in the average global surface temperature and rainfall. Diarrhoea is an important disease to study in this context because of its sensitivity to climatic parameters, and because children are particularly vulnerable to temperature variability as their immune system is not yet fully developed [
5,
6]. The primary impact of climate on society results from extreme weather events which are linked to changes in climatic variability to a greater extent than to changes in the mean values of climatic variables [
7]. In the specific case of diarrhoea, climatic variability, especially rainfall and temperature variation, more generally impact diarrhoeal incidence through their effects on the growth of the various bacteria, protozoa, viruses and helminths that cause the infections of which diarrhoea is a symptom [
6]. The effects of variation in rainfall and temperature on diarrhoea depend on the season in which the variation occurs [
8,
9].
The relationship between climate and diarrhoeal diseases is complex because of the myriad confounding variables and transmission routes that can affect the disease incidence and the fact that diarrhoea is caused by different pathogens [
10,
11,
12,
13]. Despite this complexity, evidence suggests that climatic factors, such as temperature and rainfall, are associated with the occurrence of diarrhoea; indeed an increase in temperature is associated with an increase in the incidence of diarrhoea [
14,
15,
16,
17,
18,
19]. However, previous studies exploring the effects of climatic factors on diarrhoea are inconclusive. One reason is that climatic data obtained from observing stations might contain measurement bias, as most of these monitoring sites are located in or in close proximity to urban areas, while temperature may show considerable variation even within cities [
20]. Satellite remote sensing technologies offer new opportunities because of the broad spatial coverage [
21]. To our knowledge only few studies used satellite remote sensing data to assess the effects of climatic factors on childhood diarrhoea. However, there is little evidence to support the hypothesis that the association between climatic factors and diarrhoeal incidence differs between rural and urban areas. Also, to date, no studies have assessed the relationship between climatic factors and diarrhoeal incidence using satellite remote sensing data in Senegal. This study aimed to fill these knowledge gaps. Moreover, the relationship between temperature variability and childhood diarrhoea remains to be explored, even though large temperature variability may stress children’s immune system and compromise their resistance to intestinal aetiological agents [
22].
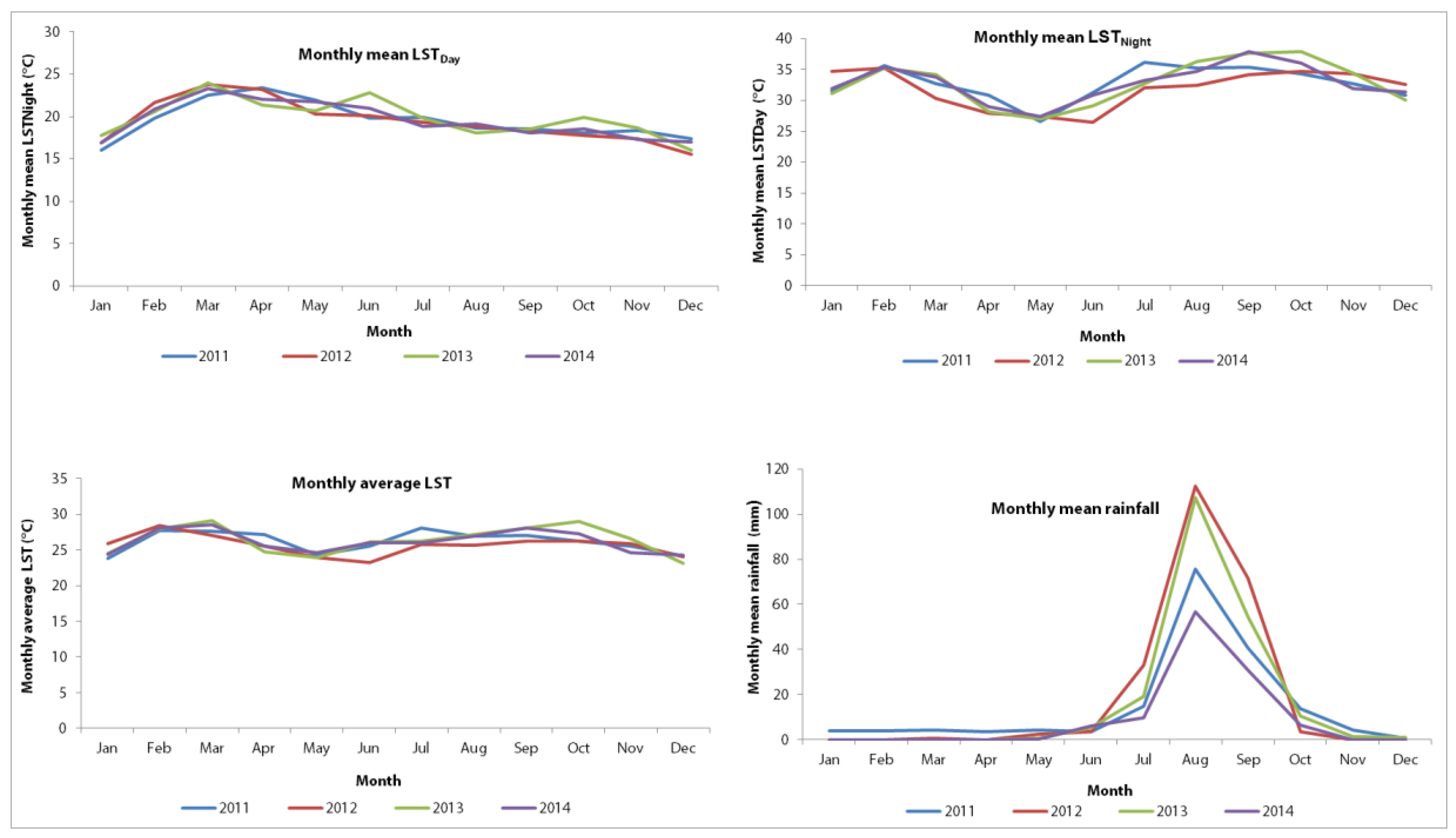
Results from a recent study conducted in Senegal showed that there is an increase in temperature during the cool season with significant inter-annual variation (–1.8 °C to 1.7 °C) and also during the warm season with a somewhat lower inter-annual variation (–1.7 °C to 1.0 °C) [
23]. The study also showed that the variation in temperatures and heavy rainfall observed in Senegal matched scenarios put forward by the Intergovernmental Panel on Climate Change (IPCC). Hence, it is important to understand climate variability as a risk factor for infectious diseases, particularly diarrhoea. Against this background, Senegal remains a vulnerable country in view of the high presence of climate-sensitive diseases, including diarrhoea, but also because of rapid urbanization and an increasing frequency of extreme whether events, such as flooding in urban areas. This means that climatic variations particularly extreme events, must be considered when safeguarding people’s health and wellbeing.
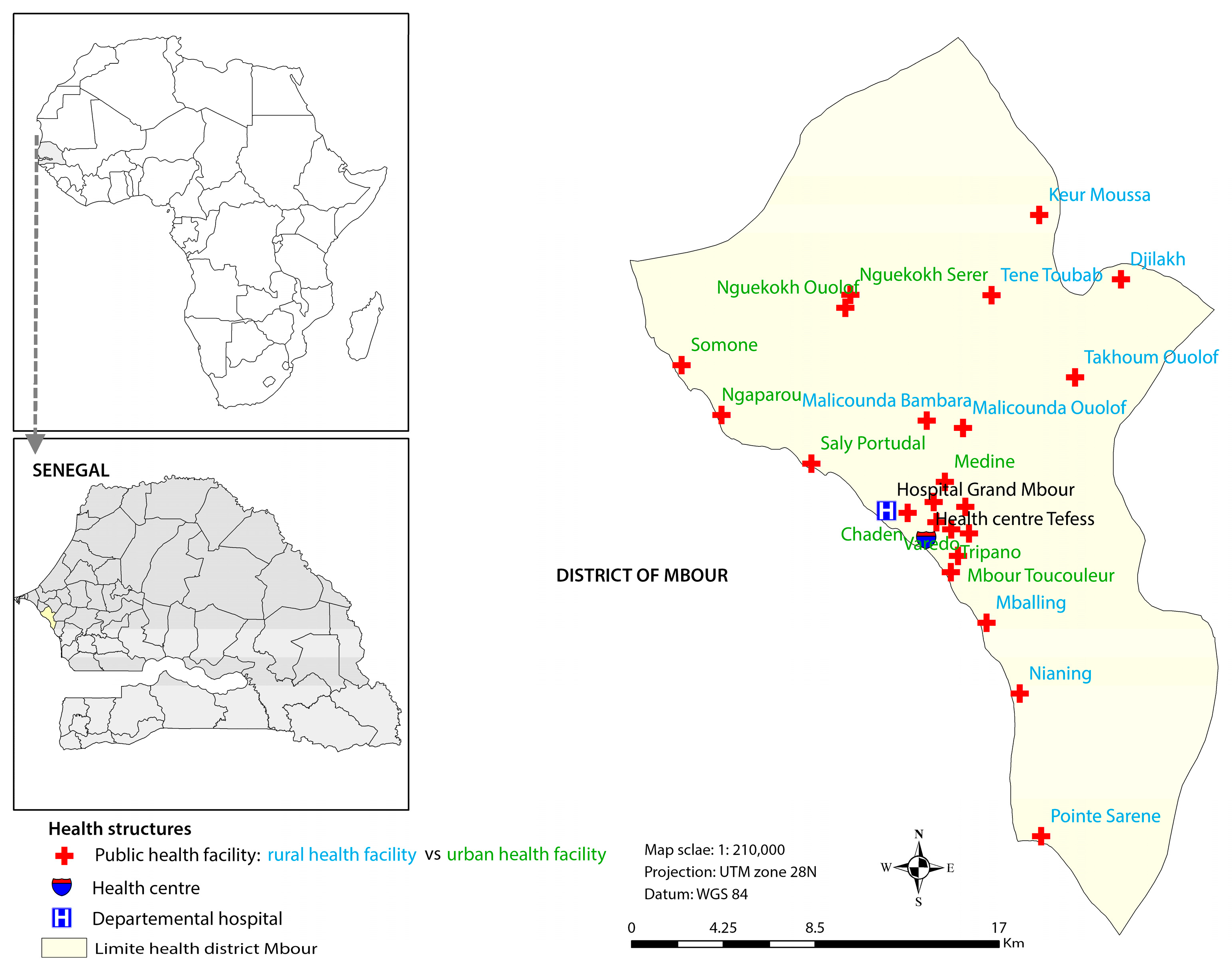
The current study focusses on the coastal secondary city of Mbour and explores the association between childhood diarrhoeal incidence and climatic factors. In this part of Senegal, the cold dry season extends from December to March and is characterized by a high diarrhoea burden. This season offers conditions that favour the rapid spread of pathogens or viruses causing diarrhoea. The objectives of the study were, firstly, to determine the incidence of diarrhoea in children under the age of five years in urban and rural settings of Mbour; secondly, to examine the seasonal patterns of diarrhoea in both areas; thirdly, to statistically assess the relationship between diarrhoea incidence and daily land surface temperature (LSTDay) and night land surface temperature (LSTNight), average temperature (LST), temperature variability (defined as the difference between temperature LSTDay and LSTNight), and rainfall; and, fourthly, to examine if the relationship with diarrhoea incidence and climatic factors differ between urban and rural settings using health surveillance data for childhood diarrhoea and satellite remote sensing data for the climate over a four-year period (January 2011 to December 2014).
4. Discussion
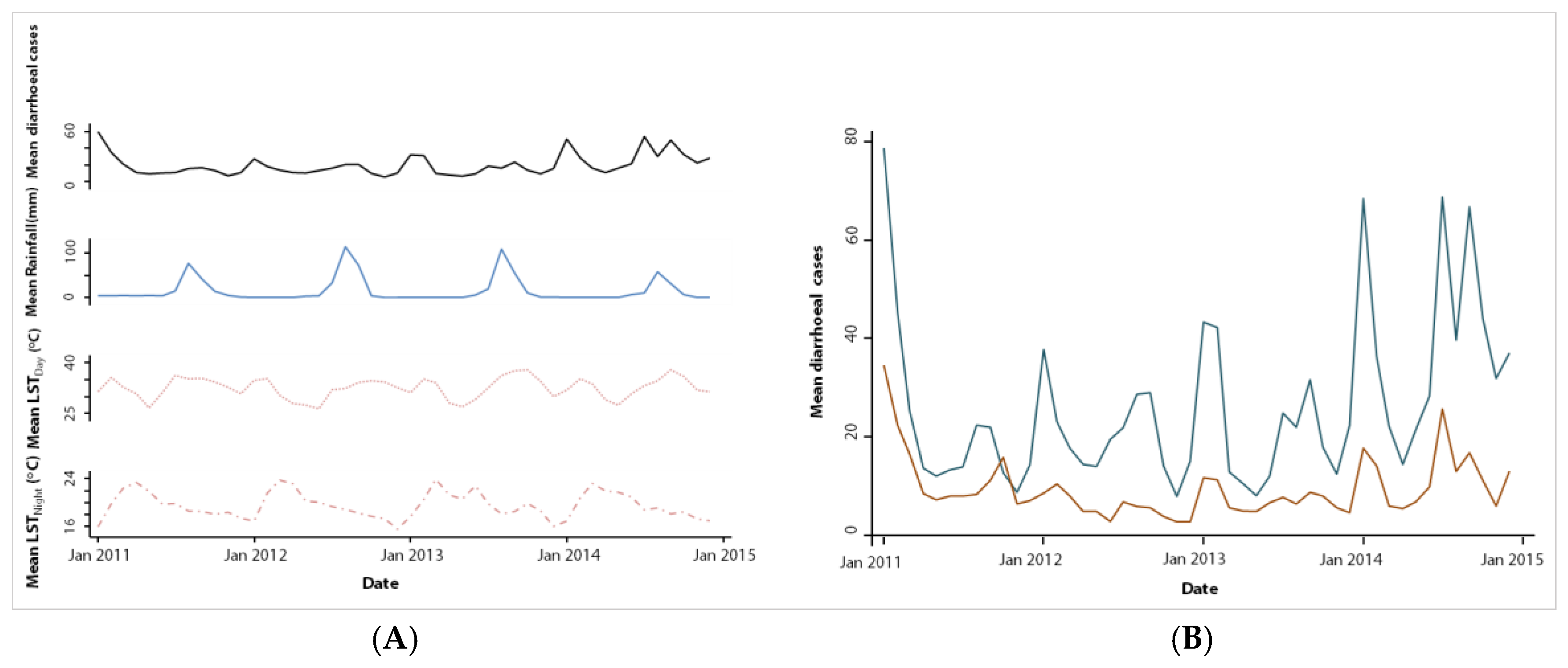
To our knowledge, this is the first study using a time-series approach to quantify the association between diarrhoeal incidence in children under five years of age and climatic factors in an entire health district of Senegal. Health-related data were readily available from the DHIS2 for the four-year study period commencing in January 2011, while remotely sensed climatic data were obtained for the same time period from various data sources. Our study allowed examining the seasonal patterns of diarrhoeal incidence in the health district of Mbour.
We found two annual peaks in diarrhoeal incidence; a first peak occurred during the cold dry season (December–March), while a second peak was observed in the rainy season (July–October). The high number of diarrhoeal cases in the cold dry season is consistent with previous studies in Senegal [
31,
32]. Indeed, prior work in Senegal revealed that the cold dry season offers conditions that favour the rapid spread of pathogens or viruses causing diarrhoea [
31,
33]. Our findings are also in line with observations made in neighbouring Guinea Bissau [
9] and Burkina Faso [
34]. Viral infections causing diarrhoea were identified in previous studies during the cold dry season in children under the age of five years across large parts of sub-Saharan Africa, including Senegal [
31,
35,
36,
37,
38,
39,
40]. These prior studies have reported prevalence rates due to rotavirus ranging from 18% to 41%. Importantly, rotaviral infections show the seasonal patterns in tropical climates [
41,
42]. Rotavirus is often the predominant aetiology of diarrhoea in infants and young children [
43], with particularly high yields of isolation during the cooler months [
44,
45]. Although this may be an important explanation for the observed higher incidence of diarrhoeal illness during the cool dry season, the present study has not specifically investigated this association.
The second peak coincided with the rainy season and generally high temperatures, which mirrors the seasonal pattern of bacterial enteric infections [
31]. We therefore speculate that during the rainy season, from July to October, the high number of diarrhoeal of infections might be driven by bacterial infection [
31,
34,
38]. In Mbour, the rainy season includes the warmest months of the year, often coupled with floods, like in other parts of Senegal. The floods are partially explained by a poor drainage system. As a result, there is enhanced human contact with wastewater, which has been associated with cholera outbreaks [
33]. Hence, it is conceivable that a large number of diarrhoeal cases during the rainy season results from an increased exposure to environmental pathogens and contaminated food due to high temperatures associated with accelerated bacterial growth [
45]. However, there might be other reasons explaining the high rates of diarrhoea in these two seasons not investigated in the current study.
We found consistent diarrhoea seasonality in children under the age of five years in both urban and rural settings of Mbour, which calls for interventions and mitigation strategies of specific times of the year. The generally higher number of diarrhoeal cases found in the urban compared to rural settings, might be explained by overcrowding and lack of timely access to quality health care services.
The study revealed that, apart from seasonality, independent effects of temperature and rainfall were also associated with diarrhoeal incidence. Previous studies already reported positive associations between diarrhoeal incidence and temperature; indeed, an increase of temperature in the short-term (monthly or weekly) was associated with an increased risk of diarrhoea [
6,
46,
47,
48]. As reported in a prior study, pathogens causing diarrhoeal diseases respond differently to temperature variability [
49]. The effect of high temperature on diarrhoeal incidence of the same month observed in the health district of Mbour may result from many factors. For instance, high temperature may lead to increased exposure to bacteria, parasites and other agents causing diarrhoea, implying that gastrointestinal diseases are more likely to occur during the hottest months of the year [
14,
50]. The associations of monthly temperatures (LST
Day, LST
Night, LST and LST variability) with diarrhoea incidence of the following month in the health district of Mbour were negative in the multivariate analyses. This suggests that the incubation period of the causative pathogen agent was shorter than one month.
Furthermore, our results from the multivariate analysis indicated that monthly mean cumulative rainfall has a positive effect on diarrhoeal incidence in the same month. Studies on the association between the risk of diarrhoea and rainfall have found contradictory results. In some cases, studies indicated that rainfall increases the risk of diarrhoea, which is in line with our observations [
18,
51,
52]. Our findings are consistent with the results of Bandyopadhya et al., who found that rainfall and the prevalence of diarrhoea were positively associated across sub-Saharan Africa [
6]. Other studies observed no association between rainfall and diarrhoea risk [
16,
19,
53], even though a US study found that any rainfall four days prior was significantly associated with an 11% increase in acute gastrointestinal illness [
54]. The positive association we found between diarrhoea and rainfall in the health district of Mbour could be explained by the fact that high rainfall can directly affect the transport of pathogens, and can affect the existing water and sanitation infrastructure, altering human exposure patterns [
55]. The transport of pathogens resulting from heavy rainfall occurs in different ways. For example, if pathogens from animal or human excreta are present in soils and on environmental surface, rainfall can mobilize theses pathogens and transport them to surface water, exposing people to pathogens [
56].
Our results showed that the effect of temperature (average LST) on diarrhoeal incidence was higher in rural compared to urban settings in the health district of Mbour but the difference was not statistically significant. In contrast, high rainfall was associated with a 31% increased risk of diarrhoea in urban settings, while there was no such association in rural settings. The reason why rainfall is a risk factor for diarrhoea in the urban setting may be due to higher levels of faecal contamination with higher exposure during the rainy season due to inexistent or unimproved sanitation system. Furthermore, in urban settings, the effects of overcrowding on diarrhoea may be exacerbated by high temperature associated with lower water availability for hygiene and sanitation. In the rural settings, high temperatures were associated with increased diarrhoeal risk, while rainfall showed no association. The negative association of LST
Day and average LST with diarrhoeal incidence of the following month observed in the urban settings is consistent with rotavirus seasonality studies [
41].
This study provides evidence that the influence of temperature on diarrhoeal risk is more pronounced in rural than in urban settings, and rainfall is more likely to increase diarrhoeal risk in urban settings of Mbour. This may be explained by the interaction between climatic factors and differences in hygiene behaviour and sanitation status in urban and rural settings. The identification of climatic factors associated with diarrhoeal seasonality in this study sheds new light on the possible role of climatic variability in the occurrence of diarrhoea. Further, the potential factors of diarrhoeal seasonality we identified will facilitate future studies assessing the impact of social and economic development on diarrhoeal diseases in Senegal.
Several limitations should be acknowledged in this study. Firstly, our analysis relies on diagnosed diarrhoeal cases at health facilities, which, we assume, were mostly moderate or severe cases. Mild diarrhoeal episodes were more likely to go unreported. It follows that the trends reported here are only applicable to moderate and severe diarrhoeal cases. Secondly, our diagnostic work-up did not allow specific diagnosis of the pathogenic agents, which restrict us to examine the seasonality of specific pathogens, which might be necessary for future vaccine programmes. Given this shortcoming, we could not specifically analyse the association between climatic factors and the causative agents of diarrhoea. Characterizing the role of climatic factors in diarrhoeal risk is challenging due to a general lack of pathogen-specific diagnoses, with “all-causes” diarrhoeal data reflecting a combination of viral, parasitic and bacterial pathogens, which vary in transmission dynamics and sensitivities to environmental conditions [
57]. With the mentioned limitations, the main strength of the current study is the use of an existing dataset (surveillance data) collected at 24 health facilities, coupled with remotely sensed climate data specific for health facility levels to assess the association between diarrhoeal diseases and climatic factors for a four-year period.