Impact of Smoking Ban on Passive Smoke Exposure in Pregnant Non-Smokers in the Southeastern United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Smoking Ban
2.3. Cotinine Collection and Assay Procedures
2.4. Assessment of Smoking Status Based on Cotinine
2.5. Assessment of Smoking Status Based on Self-Report
2.6. Assessment of Maternal Prenatal Stress and Depression
2.7. Data Analysis
3. Results
3.1. Sample Characteristics
3.2 Comparison of Self-Reported Passive Smoking Status and Cotinine Levels
3.3. Cotinine Levels and Characteristics of Non-Smoking Pregnant Women
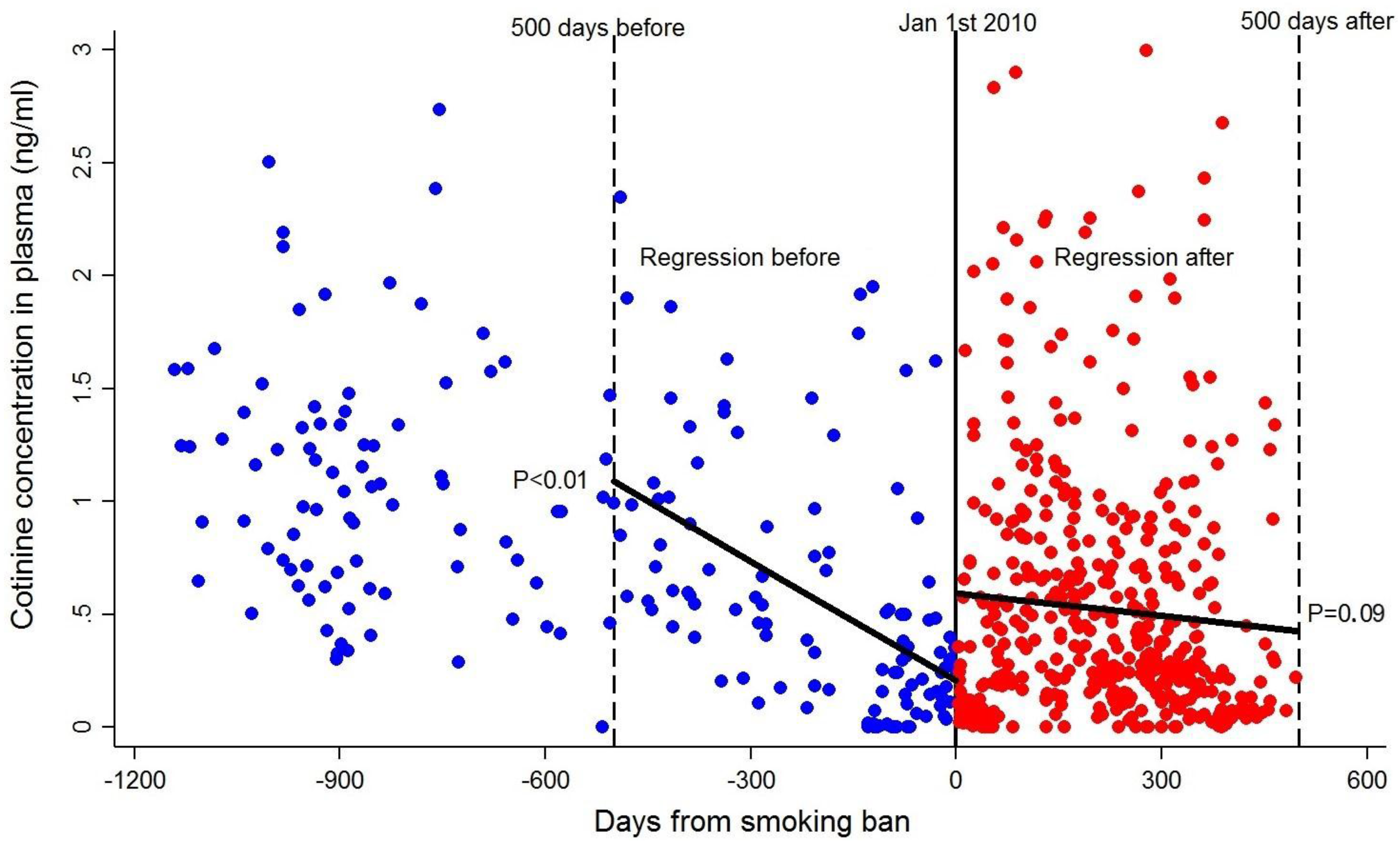
3.4. Effect of the Smoking Ban in Public Spaces in Non-Smoking Pregnant Women
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tynan, M.A.; Holmes, C.B.; Promoff, G.; Hallett, C.; Hopkins, M.; Frick, B. State and Local Comprehensive Smoke-Free Laws for Worksites, Restaurants, and Bars—United States, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gong, T.T.; Liu, C.X.; Wu, Q.J. Associations between Passive Maternal Smoking during Pregnancy and Preterm Birth: Evidence from a Meta-Analysis of Observational Studies. PLoS ONE 2016, 11, e0147848. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.; Jaakkola, N.; Zahlsen, K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environ. Health Perspect. 2011, 109, 557–561. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Britton, J.; Venn, A. Secondhand Smoke and Adverse Fetal Outcomes in Nonsmoking Pregnant Women: A Meta-Analysis. Pediatrics 2011, 127, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Surgeon General, U.S. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Surgeon General, U.S.: Washington, DC, USA, 2008.
- Windham, G.C.; Eaton, A.; Hopkins, B. Evidence for an association between environmental tobacco smoke exposure and birthweight: A meta-analysis and new data. Paediatr. Perinat. Epidemiol. 1999, 13, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Callinan, J.E.; Clarke, A.; Doherty, K.; Kelleher, C. Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Frazer, K.; Callinan, J.E.; McHugh, J.; van Baarsel, S.; Clarke, A.; Doherty, K.; Kelleher, C. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Pickett, M.S.; Schober, S.E.; Brody, D.J.; Curtin, L.R.; Giovino, G.A. Smoke-free laws and secondhand smoke exposure in US non-smoking adults, 1999–2002. Tob. Control 2006, 15, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Mannisto, T.; Bloigu, A.; Heino, A.; Gissler, M.; Surcel, H.M. Changes in objectively measured smoking in pregnancy by time and legislative changes in Finland: A retrospective cohort study. BMJ Open 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Puig, C.; Vall, O.; García-Algar, O.; Papaseit, E.; Pichini, S.; Saltó, E.; Villalbí, J.R. Assessment of prenatal exposure to tobacco smoke by cotinine in cord blood for the evaluation of smoking control policies in Spain. BMC Pregnancy Childbirth 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Caruso, C.; Perico, A.; Pacifici, R.; Monleon, T.; Garcia-Algar, O.; Rossi, S.; Pichini, S. Assessment of foetal exposure to cigarette smoke after recent implementations of smoke-free policy in Italy. Acta Paediatr. 2008, 97, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Been, J.V.; Mackay, D.F.; Millett, C.; Pell, J.P.; van Schayck, O.C.P.; Sheikh, A. Impact of smoke-free legislation on perinatal and infant mortality: A national quasi-experimental study. Sci. Rep. 2015, 5, 13020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, R.; Slejko, J.F.; Libby, A.M. A Citywide Smoking Ban Reduced Maternal Smoking and Risk for Preterm Births: A Colorado Natural Experiment. J. Womens Health 2012, 21, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Z.; Daly, S.; Clarke, V.; Keogan, S.; Clancy, L. Smoking Ban and Small-For.-Gestational Age Births in Ireland. PLoS ONE 2013, 8, e57441. [Google Scholar]
- Simón, L.; Pastor-Barriuso, R.; Boldo, E.; Fernández-Cuenca, R.; Ortiz, C.; Linares, C.; Medrano, M.J.J.; Galán, I. Smoke-Free Legislation in Spain and Prematurity. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Dimes, M.O. March of Dimes 2016 Premature Birth Report Card. March of Dimes 2017. Available online: http://www.marchofdimes.org/materials/premature-birth-report-card-united-states.pdf (accessed on 3 January 2018).
- Shipton, D.; Tappin, D.M.; Vadiveloo, T.; Crossley, J.A.; Aitken, D.A.; Chalmers, J. Reliability of self-reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- Land, T.G.; Landau, A.S.; Manning, S.E.; Purtill, J.K.; Pickett, K.; Wakschlag, L.; Dukic, V.M. Who Underreports Smoking on Birth Records: A Monte Carlo Predictive Model with Validation. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Avila-Tang, E.; Elf, J.L.; Cummings, M.K.; Fong, G.T.; Hovell, M.F.; Klein, J.D.; McMillen, R.; Winickoff, J.P.; Samet, J.M. Assessing secondhand smoke exposure with reported measures. Tob. Control 2013, 22, 156–163. [Google Scholar] [CrossRef] [PubMed]
- DeLorenze, G.N.; Kharrazi, M.; Kaufman, F.L.; Eskenazi, B.; Bernert, J.T. Exposure to Environmental Tobacco Smoke in Pregnant Women: The Association between Self-Report and Serum Cotinine. Environ. Res. 2002, 90, 21–32. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Granath, F.; Johansson, A.L.V.; Cnattingius, S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006, 85, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Swamy, G.K.; Reddick, K.L.B.; Brouwer, R.J.N.; Pollak, K.I.; Myers, E.R. Smoking prevalence in early pregnancy: Comparison of self-report and anonymous urine cotinine testing. J. Matern.-Fetal Neonatal Med. 2010, 24, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital signs: Nonsmokers’ exposure to secondhand smoke—United States, 1999–2008. Morb. Mortal. Wkly. Rep. 2010, 59, 1141–1146. [Google Scholar]
- Hawkins, S.S.; Dacey, C.; Gennaro, S.; Keshinover, T.; Gross, S.; Gibeau, A.; Lulloff, A.; Aldous, K.M. Secondhand Smoke Exposure among Nonsmoking Pregnant Women in New York City. Nicotine Tob. Res. 2014, 16, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Patelarou, E.; Chatzi, L.; Roumeliotaki, T.; Sarri, K.; Murphy, S.; Koutis, A.; Kafatos, A.G.; Kogevinas, M. Factors Associated with Active Smoking, Quitting, and Secondhand Smoke Exposure among Pregnant Women in Greece. J. Epidemiol. 2010, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Mannino, D.M.; Jemal, A. Socioeconomic disparities in secondhand smoke exposure among US never-smoking adults: The National Health and Nutrition Examination Survey 1988–2010. Tob. Control 2014, 24, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, J.L.; Bernert, J.T.; Caudill, S.P.; Sosnoff, C.S.; Pechacek, T.F. Trends in the Exposure of Nonsmokers in the U.S. Population to Secondhand Smoke: 1988–2002. Environ. Health Perspect. 2006, 114, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Levine, R.J.; Clemens, J.D.; DerSimonian, R.; Wilkins, D.G. Serum cotinine concentration and self-reported smoking during pregnancy. Am. J. Epidemiol. 1998, 148, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Althabe, F.; Onyamboko, M.; Kaseba-Sata, C.; Castilla, E.E.; Freire, S.; Garces, A.L.; Parida, S.; Goudar, S.S.; Kadir, M.; et al. Tobacco Use and Secondhand Smoke Exposure during Pregnancy: An Investigative Survey of Women in 9 Developing Nations. Am. J. Public Health 2008, 98, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Courtney, L.P.; El-Mohandes, A.A.E.; Gantz, M.G.; Blake, S.M.; Thornberry, J.; El-Khorazaty, N.M.; Perry, D.; Kiely, M. Relationships between Self-Reported Smoking, Household Environmental Tobacco Smoke Exposure and Depressive Symptoms in a Pregnant Minority Population. Matern. Child Health J. 2011, 15, S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Mbah, A.K.; Salihu, H.M.; Dagne, G.; Wilson, R.E.; Bruder, K. Exposure to environmental tobacco smoke and risk of antenatal depression: Application of latent variable modeling. Arch. Women Ment. Health 2013, 16, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Forman, M.R.; Calingaert, B.; Demark-Wahnefried, W.; Kurtzberg, J.; Jirtle, R.L.; Murphy, S.K. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 2011, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Murphy, S.K.; Murtha, A.P.; Fuemmeler, B.F.; Schildkraut, J.; Huang, Z.; Overcash, F.; Kurtzberg, J.; Jirtle, R.; Iversen, E.S.; et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics 2012, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Murphy, S.K.; Adigun, A.; Huang, Z.; Overcash, F.; Wang, F.; Jirtle, R.L.; Schildkraut, J.M.; Murtha, A.P.; Iversen, E.S.; Hoyo, C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene 2012, 494, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Lee, C.T.; Soubry, A.; Iversen, E.S.; Huang, Z.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Murphy, S.K.; Hoyo, C. DNA Methylation of Regulatory Regions of Imprinted Genes at Birth and Its Relation to Infant Temperament. Genet. Epigenet. 2016, 8, 59–67. [Google Scholar] [CrossRef] [PubMed]
- North Carolina Department of Health and Human Services. Evaluation of the NC Smoke-free Restaurants and Bars Law; North Carolina Department of Health and Human Services: Raleigh, NC, USA, 2013.
- Dempsey, D.A.; Meyers, M.J.; Oh, S.S.; Nguyen, E.A.; Fuentes-Afflick, E.; Wu, A.H.; Jacob, P.; Benowitz, N.L. Determination of Tobacco Smoke Exposure by Plasma Cotinine Levels in Infants and Children Attending Urban. Public Hospital Clinics. Arch. Pediatr. Adolesc. Med. 2012, 166, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Bernert, J.T.; Jacob, P.; Holiday, D.B.; Benowitz, N.L.; Sosnoff, C.S.; Doig, M.V.; Feyerabend, C.; Aldous, K.M.; Sharifi, M.; Kellogg, M.D.; et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: A round-robin study. Nicotine Tob. Res. 2009, 11, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Yu, L.; Duan, M.; Ramos, L.; Yturralde, O.; Benowitz, N.L. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography–tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J. Chromatogr. B 2011, 879, 267–276. [Google Scholar]
- Benowitz, N.L.; Dains, K.M.; Dempsey, D.; Wilson, M.; Jacob, P. Racial Differences in the Relationship between Number of Cigarettes Smoked and Nicotine and Carcinogen Exposure. Nicotine Tob. Res. 2011, 13, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Daniels, J.L.; Poole, C.; Olshan, A.F.; Hornung, R.; Bernert, J.T.; Khoury, J.; Needham, L.L.; Barr, D.B.; Lanphear, B.P. Prenatal environmental tobacco smoke exposure and early childhood body mass index. Paediatr. Perinat. Epidemiol. 2010, 24, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Williamson, G.M. Perceived Stress in a Probability Sample of the United States; Sage: Thousand Oaks, CA, USA, 1988. [Google Scholar]
- Ickovics, J.R.; Kershaw, T.S.; Westdahl, C.; Magriples, U.; Massey, Z.; Reynolds, H.; Rising, S.S. Group prenatal care and perinatal outcomes: A randomized controlled trial. Obstet. Gynecol. 2007, 110 Pt 1, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Dole, N.; Savitz, D.A.; Hertz-Picciotto, I.; Siega-Riz, A.M.; McMahon, M.J.; Buekens, P. Maternal Stress and Preterm Birth. Am. J. Epidemiol. 2003, 157, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, P.M.; Seeley, J.R.; Roberts, R.E.; Allen, N.B. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 1997, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004, 6, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.; Adams, E.K.; Dietz, P.M.; Tong, V.T.; Kannan, V. Tobacco Control. Policies, Birth Outcomes, and Maternal Human Capital. J. Hum. Cap. 2013, 7, 130–160. [Google Scholar] [CrossRef]
- McGeary, K.A.; Dave, D.M.; Lipton, B.J.; Roeper, T. Impact of Comprehensive Smoking Bans on the Health of Infants and Children. Bull. Aging Health 2017, 11, 23995. [Google Scholar]
- Center for Public Health Systems Science. Point-of-Sale Strategies: A Tobacco Control Guide; Center for Public Health Systems Science: St. Louis, MO, USA, 2014. [Google Scholar]
- Tobacco use among U.S. racial/ethnic minority groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary. Morb. Mortal. Wkly. Rep. 1998, 47, 1–16.
- Perez-Stable, E.J.; Herrera, B.; Jacob, P., III; Benowitz, N.L. Nicotine Metabolism and Intake in Black and White Smokers. JAMA 1998, 280, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Dole, N.; Terry, J.W., Jr.; Zhou, H.; Thorp, J.M., Jr. Smoking and pregnancy outcome among African-American and white women in central North. Carolina. Epidemiology 2001, 12, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, T.K.; Spencer, S.M.; Hoskinson, R.A., Jr.; Sachs, D.P.; Garvey, A.J. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob. Res. 2005, 7, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Florescu, A.; Ferrence, R.; Einarson, T.; Selby, P.; Soldin, O.; Koren, G. Methods for Quantification of Exposure to Cigarette Smoking and Environmental Tobacco Smoke: Focus on Developmental Toxicology. Ther. Drug Monit. 2009, 31, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.R.A.; Brinthaupt, J.; Stehle, J.M.; James, G.D. Comparison of Self-Reported Smoking and Urinary Cotinine Levels in a Rural Pregnant Population. J. Obstet. Gynecol. Neonatal Nurs. 2004, 33, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.R.; Windsor, R.A.; Perkins, L.L.; Lowe, J.B. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Matern. Child Health J. 1998, 2, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.J.; Wen, X.Z.; Ding, P.; He, Y.H.; Xie, C.B.; Liu, T.; Lin, J.M.; Yuan, S.X.; Guo, X.L.; Jia, D.Q.; et al. Interaction between Maternal Passive Smoking during Pregnancy and CYP1A1 and GSTs Polymorphisms on Spontaneous Preterm Delivery. PLoS ONE 2012, 7, e49155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Full Sample | All Non-Smokers | Non-Smokers Pre-Ban | Non-Smokers Post-Ban | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Race | ||||
| African American | 452 (53.1) | 336 (50.3) | 89 (44.5) | 247 (52.8) |
| Caucasian | 332 (39.0) | 274 (41.0) | 99 (49.5) | 175 (37.4) |
| Hispanic/Other | 68 (8.0) | 58 (8.7) | 12 (6.0) | 46 (9.8) |
| Marital Status | ||||
| Never married | 263 (30.9) | 184 (27.5) | 48 (24.0) | 136 (29.1) |
| Married | 380 (44.6) | 346 (51.8) | 111 (55.5) | 235 (50.2) |
| Living with partner | 138 (16.2) | 91 (13.6) | 28 (14.0) | 63 (13.5) |
| Divorced/separated | 28 (3.3) | 19 (2.8) | 10 (5.0) | 9 (1.9) |
| Other | 16 (1.9) | 9 (1.3) | 1 (.5) | 8 (1.7) |
| Education | ||||
| <High school | 120 (14.1) | 61 (9.1) | 10 (5.0) | 51 (10.9) |
| High school/GED | 177 (20.8) | 125 (18.7) | 45 (22.5) | 80 (17.1) |
| Some college | 198 (23.2) | 145 (21.7) | 50 (25.0) | 95 (20.3) |
| College graduate | 332 (39.0) | 319 (47.8) | 93 (46.5) | 226 (48.3) |
| No college | 297 (34.9) | 186 (27.8) | 55 (27.5) | 131 (28.0) |
| Any college | 5030 (62.2) | 464 (69.5) | 143 (71.5) | 321 (68.6) |
| Parity | ||||
| 0 | 320 (37.6) | 270 (40.4) | 58 (29.0) | 212 (45.3) |
| 1 | 288 (33.8) | 222 (33.2) | 71 (35.5) | 151 (32.3) |
| 2 | 140 (16.4) | 104 (15.6) | 44 (22.0) | 60 (12.8) |
| 3 | 48 (5.6) | 34 (5.1) | 11 (5.5) | 23 (4.9) |
| ≥4 | 45 (5.3) | 27 (4.0) | 12 (6.0) | 15 (3.2) |
| Full Sample | All Non-Smokers | Non-Smokers Pre-Ban | Non-Smokers Post-Ban | |
|---|---|---|---|---|
| Cotinine (Mean, SD) | Cotinine (Mean, SD) | Cotinine (Mean, SD) | Cotinine (Mean, SD) | |
| Race | ||||
| African American | 19.21 (53.25) | 0.68 (0.65) | 0.92 (0.65) | 0.59 (0.62) |
| Caucasian | 13.65 (39.57) | 0.52 (0.48) | 0.63 (0.51) | 0.46 (0.45) |
| Hispanic/Other | 6.93 (34.87) | 0.50 (0.58) | 0.91 (0.73) | 0.39 (0.48) |
| Marital Status | ||||
| Never married | 21.82 (55.86) | 0.67 (0.65) | 0.85 (0.65) | 0.61 (0.64) |
| Married | 6.61 (30.11) | 0.52 (0.50) | 0.68 (0.54) | 0.44 (0.46) |
| Living with partner | 19.70 (42.81) | 0.72(0.69) | 1.03 (0.77) | 0.58 (0.61) |
| Divorced/separated | 29.54 (59.33) | 0.77 (0.45) | 0.81 (0.34) | 0.74 (0.56) |
| Other | 57.15 (91.21) | 0.61 (0.67) | 0.01 (--) | 0.68 (0.68) |
| Education | ||||
| <High school | 42.53 (76.19) | 0.73 (0.75) | 0.75 (0.50) | 0.73 (0.79) |
| High school/GED | 24.41 (60.37) | 0.72 (0.64) | 0.99 (0.67) | 0.57 (0.57) |
| Some college | 16.79 (41.31) | 0.62 (0.58) | 0.73 (0.61) | 0.56 (0.56) |
| College graduate | 1.55 (9.61) | 0.51 (0.50) | 0.69 (0.56) | 0.44 (0.45) |
| No college | 31.73 (67.67) | 0.72 (0.67) | 0.95 (0.64) | 0.63 (0.67) |
| Any college | 7.25 (27.37) | 0.55 (0.53) | 0.71 (0.58) | 0.47 (0.49) |
| Parity | ||||
| 0 | 8.12 (29.01) | 0.56 (0.58) | 0.77 (0.66) | 0.51 (0.55) |
| 1 | 14.86 (45.80) | 0.58 (0.54) | 0.68 (0.55) | 0.53 (0.53) |
| 2 | 21.66 (49.32 | 0.62 (0.62) | 0.84 (0.62) | 0.46 (0.58) |
| 3 | 34.58 (79.11) | 0.71 (0.56) | 0.80 (0.52) | 0.67 (0.59) |
| ≥4 | 46.70 (82.56) | 0.75 (0.59) | 1.01 (0.59) | 0.53 (0.50) |
| Self-Reported Passive Exposure | |||
|---|---|---|---|
| No Exposure | Passive Exposure | Total | |
| Cotinine values | |||
| No Exposure (<1.0 ng/mL) | 382 | 50 | 432 |
| Passive Exposure (1–3 ng/mL) | 51 | 20 | 71 |
| Total | 433 | 70 | 503 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schechter, J.C.; Fuemmeler, B.F.; Hoyo, C.; Murphy, S.K.; Zhang, J.; Kollins, S.H. Impact of Smoking Ban on Passive Smoke Exposure in Pregnant Non-Smokers in the Southeastern United States. Int. J. Environ. Res. Public Health 2018, 15, 83. https://doi.org/10.3390/ijerph15010083
Schechter JC, Fuemmeler BF, Hoyo C, Murphy SK, Zhang J, Kollins SH. Impact of Smoking Ban on Passive Smoke Exposure in Pregnant Non-Smokers in the Southeastern United States. International Journal of Environmental Research and Public Health. 2018; 15(1):83. https://doi.org/10.3390/ijerph15010083
Chicago/Turabian StyleSchechter, Julia C., Bernard F. Fuemmeler, Cathrine Hoyo, Susan K. Murphy, Junfeng (Jim) Zhang, and Scott H. Kollins. 2018. "Impact of Smoking Ban on Passive Smoke Exposure in Pregnant Non-Smokers in the Southeastern United States" International Journal of Environmental Research and Public Health 15, no. 1: 83. https://doi.org/10.3390/ijerph15010083
APA StyleSchechter, J. C., Fuemmeler, B. F., Hoyo, C., Murphy, S. K., Zhang, J., & Kollins, S. H. (2018). Impact of Smoking Ban on Passive Smoke Exposure in Pregnant Non-Smokers in the Southeastern United States. International Journal of Environmental Research and Public Health, 15(1), 83. https://doi.org/10.3390/ijerph15010083




