Physiological Effects of Visual Stimulation with Forest Imagery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
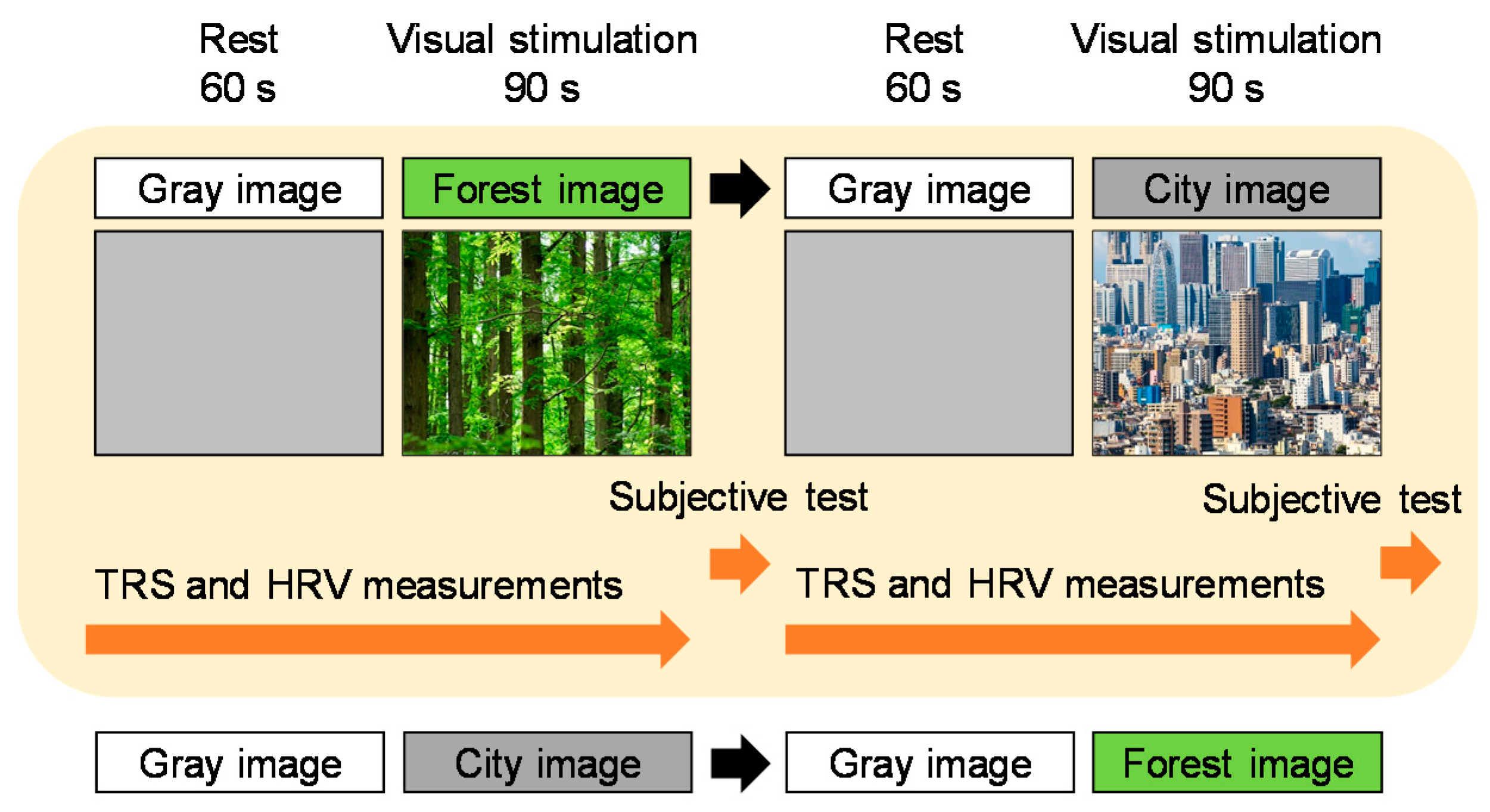
2.2. Visual Stimulation
2.3. Study Protocol
2.4. Physiological Measurement
2.4.1. Near-Infrared Time-Resolved Spectroscopy (TRS)
2.4.2. Heart Rate Variability (HRV) and Heart Rate
2.5. Psychological Measurement
2.6. Statistical Analysis
3. Results
3.1. Physiological Effects
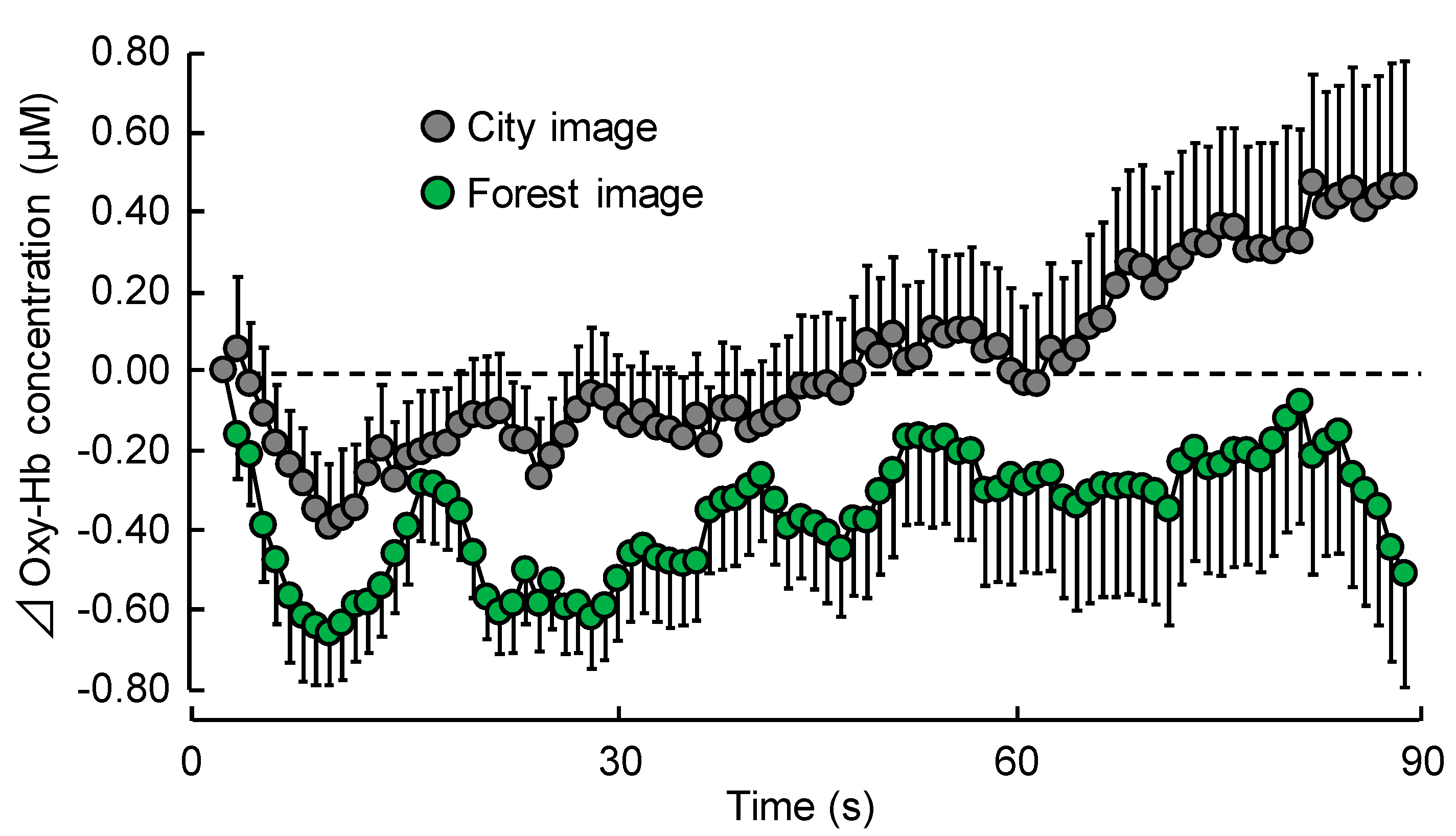
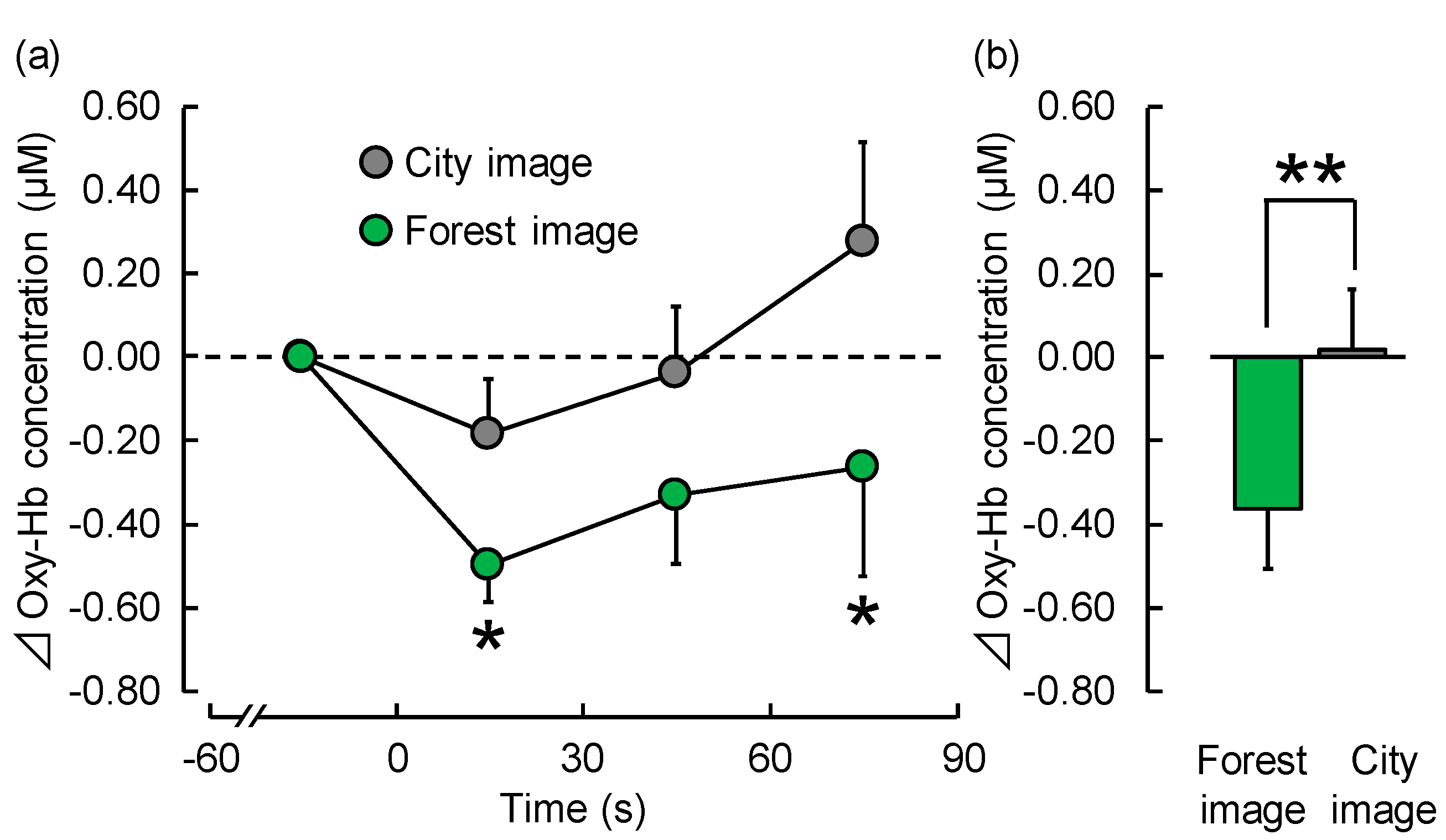
3.1.1. TRS
3.1.2. HRV and Heart Rate
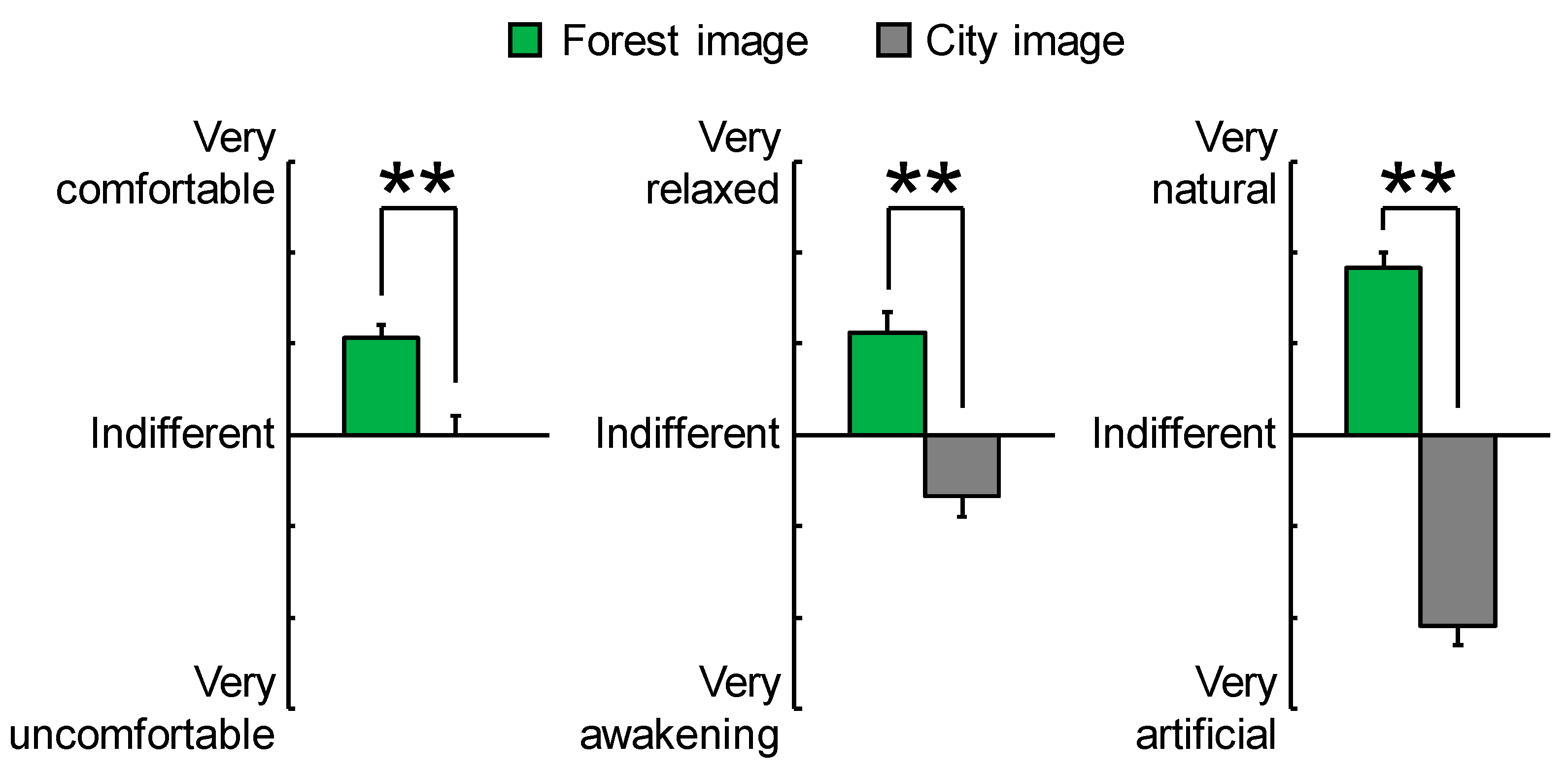
3.2. Psychological Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dye, C. Health and urban living. Science 2008, 319, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Nations United. World Urbanization Prospects: The 2014 Revision, Highlights. Department of Economic and Social Affairs; Population Division: New York, NY, USA, 2014. [Google Scholar]
- Vlahov, D.; Freudenberg, N.; Proietti, F.; Ompad, D.; Quinn, A.; Nandi, V.; Galea, S. Urban as a determinant of health. J. Urban Health 2007, 84, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takano, T.; Nakamura, K.; Takeuchi, S. Health level influenced by urban residential conditions in a megacity—Tokyo. Urban Stud. 1996, 33, 879–894. [Google Scholar] [CrossRef]
- Peen, J.; Schoevers, R.A.; Beekman, A.T.; Dekker, J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr. Scand. 2010, 121, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Murray, A.L.; Booth, T. Do urban environments increase the risk of anxiety, depression and psychosis? An epidemiological study. J. Affect. Disord. 2013, 150, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Mantlera, A.; Logan, A.C. Natural environments and mental health. Adv. Integr. Med. 2015, 2, 5–12. [Google Scholar] [CrossRef]
- Craig, J.M.; Logan, A.C.; Prescott, S.L. Natural environments, nature relatedness and the ecological theatre: Connecting satellites and sequencing to shinrin-yoku. J. Physiol. Anthropol. 2016, 35. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effects of wood on humans: A review. J. Wood Sci. 2017, 63, 1–23. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-yoku (forest bathing) and nature therapy: A state-of-the-art review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Selhub, E.M.; Logan, A.C. Your Brain on Nature: The Science of Nature’s Influence on Your Health, Happiness and Vitality; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological effects of “Shinrin-yoku” (taking in the atmosphere of the forest) in an old-growth broadleaf forest in Yamagata prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. The restorative effects of viewing real forest landscapes: Based on a comparison with urban landscapes. Scand. J. For. Res. 2009, 24, 227–234. [Google Scholar] [CrossRef]
- Park, B.J.; Kasetani, T.; Morikawa, T.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. Physiological effects of forest recreation in a young conifer forest in Hinokage Town, Japan. Silva Fenn. 2009, 43, 291–301. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Lee, J.; Park, B.J.; Tyrväinen, L.; Kagawa, T.; Miyazaki, Y. Physiological and psychological effects of viewing urban forest landscapes assessed by multiple measurements. Landsc. Urban Plan. 2013, 113, 90–93. [Google Scholar] [CrossRef]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid. Based Complement. Altern. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, Y.; et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Yabunaka, N.; Takayama, S. Shinrin-Yoku (forest-air bathing and walking) effectively decreases blood glucose levels in diabetic patients. Int. J. Biometeorol. 1998, 41, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.X.; Lan, X.G.; Cao, Y.B.; Chen, Z.M.; He, Z.H.; Lv, Y.D.; Wang, Y.Z.; Hu, X.L.; Wang, G.F.; Yan, J. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province. China Biomed. Environ. Sci. 2012, 25, 317–324. [Google Scholar] [PubMed]
- Sung, J.; Woo, J.M.; Kim, W.; Lim, S.K.; Chung, E.J. The effect of cognitive behavior therapy-based “forest therapy” program on blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin. Exp. Hypertens. 2012, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, D.C. Cardiac and pulmonary benefits of forest walking versus city walking in elderly women: A randomised, controlled, open-label trial. Eur. J. Integr. Med. 2014, 6, 5–11. [Google Scholar] [CrossRef]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al. Physiological and psychological effects of forest therapy on middle-age males with high-normal blood pressure. Int. J. Environ. Res. Public Health 2015, 12, 2521–2531. [Google Scholar]
- Song, C.; Ikei, H.; Kobayashi, M.; Miura, T.; Taue, M.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Effect of forest walking on autonomic nervous system activity in middle-aged hypertensive individuals. Int. J. Environ. Res. Public Health 2015, 12, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.H.; Chang, M.C.; Lee, S. The effects of forest therapy on depression and anxiety in patients with chronic stroke. Int. J. Neurosci. 2017, 127, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Kobayashi, M.; Miura, T.; Li, Q.; Kagawa, T.; Kumeda, S.; Imai, M.; Miyazaki, Y. Effect of viewing forest landscape on middle-aged hypertensive men. Urban For. Urban Green. 2017, 21, 247–252. [Google Scholar] [CrossRef]
- Morita, E.; Fukuda, S.; Nagano, J.; Hamajima, N.; Yamamoto, H.; Iwai, Y.; Nakashima, T.; Ohira, H.; Shirakawa, T. Psychological effects of forest environments on healthy adults: Shinrin-Yoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health 2007, 121, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Furuya, K.; Kasetani, T.; Takayama, N.; Kagawa, T.; Miyazaki, Y. Relationship between psychological responses and physical environments in forest settings. Landsc. Urban Plan. 2011, 102, 24–32. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Motohashi, Y.; Kobayashi, S. Changes in mood by inhalation of essential oils in humans II. Effect of essential oils on blood pressure, heart rate, R–R intervals, performance, sensory evaluation and POMS. Mokuzai Gakkaishi 1992, 38, 909–913. (In Japanese) [Google Scholar]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2015, 34, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Ohira, T.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Physiological effects of visual, olfactory, auditory, and tactile factors in the forest environment. In Forest Medicine; Li, Q., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 169–181. [Google Scholar]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Joung, D.; Song, C.; Ikei, H.; Okuda, T.; Igarashi, M.; Koizumi, H.; Park, B.J.; Yamaguchi, T.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of olfactory stimulation with d-limonene. Adv. Hortic. Sci. 2014, 28, 90–94. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Lostio, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Ohmae, E.; Ouchi, Y.; Oda, M.; Suzuki, T.; Nobesawa, S.; Kanno, T.; Yoshikawa, E.; Futatsubashi, M.; Ueda, Y.; Okada, H.; et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: Correlation with simultaneous positron emission tomography measurements. Neuroimage 2006, 29, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, E.; Oda, M.; Suzuki, T.; Yamashita, Y.; Kakihana, Y.; Matsunaga, A.; Kanmura, Y.; Tamura, M. Clinical evaluation of time-resolved spectroscopy by measuring cerebral hemodynamics during cardiopulmonary bypass surgery. J. Biomed. Opt. 2007, 12, 062112. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Kobayashi, N.; Tamura, M. Interpretation of near infrared spectroscopy signals: A study with a newly developed perfused rat brain model. J. Appl. Physiol. 2001, 90, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Kobayashi, H.; Ishibashi, K.; Noguchi, H. Heart rate variability; an index for monitoring and analyzing human autonomic activities. Appl. Hum. Sci. 1999, 18, 53–59. [Google Scholar] [CrossRef]
- Kanaya, N.; Hirata, N.; Kurosawa, S.; Nakayama, M.; Namiki, A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology 2003, 98, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- Victor, A.; Elsässer, A.; Hommel, G.; Blettner, M. Judging a plethora of p-values: How to contend with the problem of multiple testing. Part 10 of a series on evaluation of scientific publications. Dtsch. Arztebl. Int. 2010, 107, 50–56. [Google Scholar] [PubMed]
- Eichstaedt, K.E.; Kovatch, K.; Maroof, D.A. A less conservative method to adjust for familywise error rate in neuropsychological research: The Holm’s sequential Bonferroni procedure. NeuroRehabilitation 2013, 32, 693–696. [Google Scholar] [PubMed]
- Joung, D.; Kim, G.; Choi, Y.; Lim, H.; Park, S.; Woo, J.M.; Park, B.J. The prefrontal cortex activity and psychological effects of viewing forest landscapes in autumn season. Int. J. Environ. Res. Public Health 2015, 12, 7235–7243. [Google Scholar] [CrossRef] [PubMed]
- Kahn, P.H.; Friedman, B.; Gill, B.; Hagman, J.; Severson, R.L.; Freier, N.G.; Feldman, E.N.; Carrère, S.; Stolyar, A. A plasma display window?—The shifting baseline problem in a technologically mediated natural world. J. Environ. Psychol. 2008, 28, 192–199. [Google Scholar] [CrossRef]


| Physiological Measurement (n = 17) | Forest Image | City Image | p-Value | Significance Level |
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | |||
| µM | µM | |||
| Oxy-Hb concentration in the right prefrontal cortex | ||||
| 1–30 s | −0.50 ± 0.09 | −0.19 ± 0.13 | 0.024 | * p < 0.05 |
| 31–60 s | −0.33 ± 0.16 | −0.04 ± 0.16 | 0.097 | NS |
| 61–90 s | −0.26 ± 0.26 | 0.27 ± 0.24 | 0.011 | * p < 0.05 |
| Overall mean | −0.36 ± 0.14 | 0.02 ± 0.14 | 0.006 | ** p < 0.01 |
| Oxy-Hb concentration in the left prefrontal cortex | ||||
| 1–30 s | −0.31 ± 0.09 | −0.13 ± 0.14 | 0.131 | NS |
| 31–60 s | −0.26 ± 0.16 | −0.10 ± 0.18 | 0.260 | NS |
| 61–90 s | −0.02 ± 0.27 | 0.37 ± 0.30 | 0.087 | NS |
| Overall mean | −0.20 ± 0.16 | 0.05 ± 0.18 | 0.112 | NS |
| Psychological Measurement (n = 17) | Forest Image | City Image | pValue | Significance Level |
| Mean ± SE | Mean ± SE | |||
| Score | Score | |||
| “Comfortable” feeling | 2.1 ± 0.3 | 0.0 ± 0.4 | <0.001 | ** p < 0.01 |
| “Relaxed” feeling | 2.2 ± 0.4 | −1.4 ± 0.4 | <0.001 | ** p < 0.01 |
| “Natural” feeling | 3.6 ± 0.3 | −4.2 ± 0.4 | <0.001 | ** p < 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Ikei, H.; Miyazaki, Y. Physiological Effects of Visual Stimulation with Forest Imagery. Int. J. Environ. Res. Public Health 2018, 15, 213. https://doi.org/10.3390/ijerph15020213
Song C, Ikei H, Miyazaki Y. Physiological Effects of Visual Stimulation with Forest Imagery. International Journal of Environmental Research and Public Health. 2018; 15(2):213. https://doi.org/10.3390/ijerph15020213
Chicago/Turabian StyleSong, Chorong, Harumi Ikei, and Yoshifumi Miyazaki. 2018. "Physiological Effects of Visual Stimulation with Forest Imagery" International Journal of Environmental Research and Public Health 15, no. 2: 213. https://doi.org/10.3390/ijerph15020213






