The Effect of the eHealth Intervention ‘MyPlan 1.0’ on Physical Activity in Adults Who Visit General Practice: A Quasi-Experimental Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements
2.3. eHealth Intervention ‘MyPlan1.0’
2.4. Procedure
2.5. Statistical Analyses
3. Results
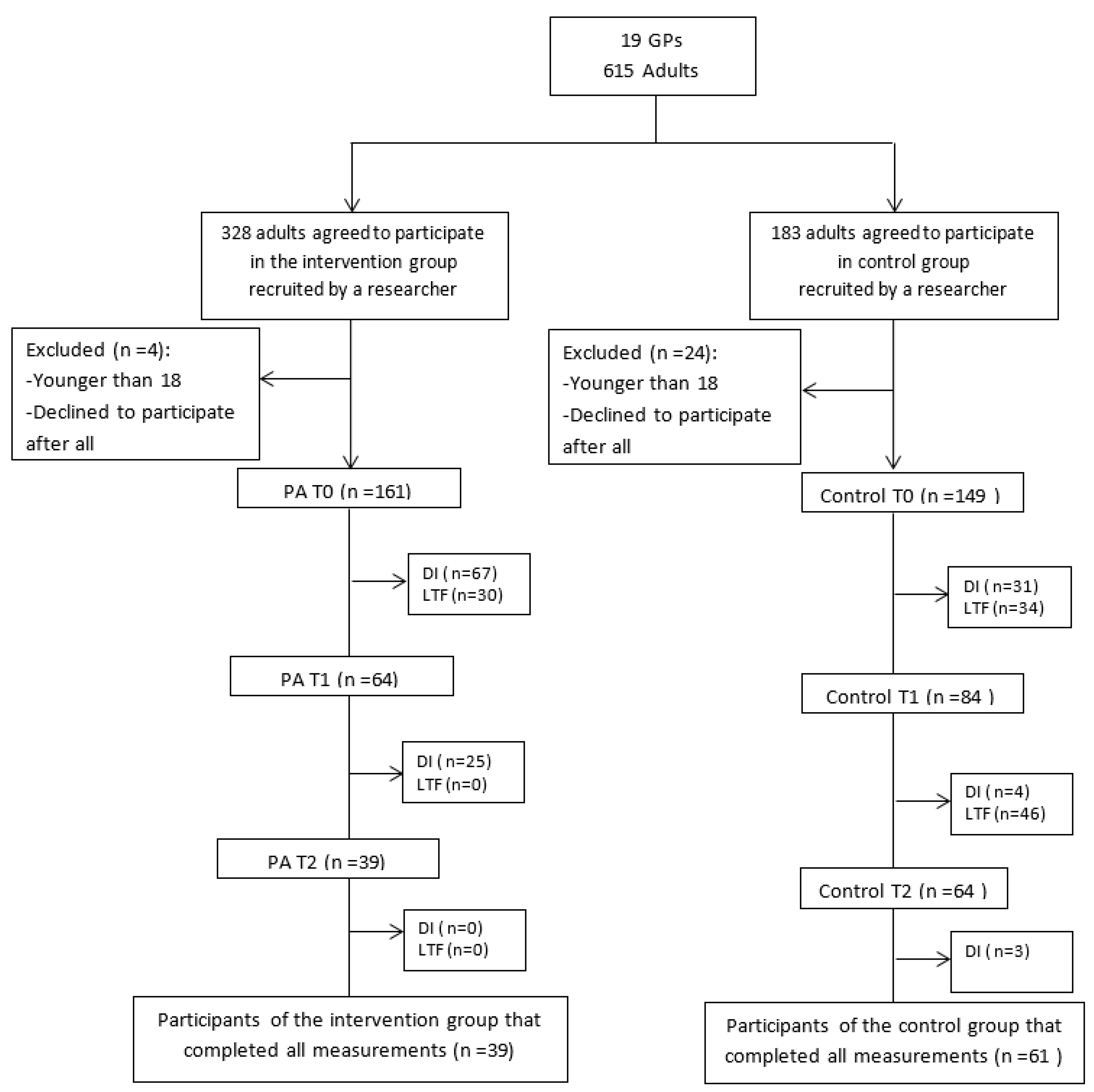
3.1. Participants Characteristics, Response and Dropout Analysis
3.2. Effects on PA
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| IPAQ | International Physical Activity Questionnaire |
| PA | physical activity |
| VPA | vigorous physical activity |
| MVPA | moderate to vigorous physical activity |
| GP | general practitioner |
References
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the american college of sports medicine and the american heart association. Med. Sci. Sports Exerc. 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Gordon, B.; Wang, Z.; Luo, X. Chronic disease and the link to physical activity. J. Sport Health Sci. 2013, 2, 3–11. [Google Scholar] [CrossRef]
- Tafforeau, J. Lichaamsbeweging. In Gezondheidsenquête (Health Interview Survey) België (Belgium); WIV-ISP: Brussels, Belgium, 2008. [Google Scholar]
- Spittaels, H.; De Bourdeaudhuij, I.; Vandelanotte, C. Evaluation of a website-delivered computer-tailored intervention for increasing physical activity in the general population. Prev. Med. 2007, 44, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Eng, T.R. The eHealth Landscape: A Terrain Map of Emerging Information and Communication Technologies in Health and Health Care; The Robert Wood Johnson Foundation: Princeton, NJ, USA, 2001. [Google Scholar]
- Broekhuizen, K.; Kroeze, W.; van Poppel, M.N.; Oenema, A.; Brug, J. A systematic review of randomized controlled trials on the effectiveness of computer-tailored physical activity and dietary behaviour promotion programs: An update. Ann. Behav. Med. 2012, 44, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Lustria, M.L.; Cortese, J.; Noar, S.M.; Glueckauf, R.L. Computer-tailored health interventions delivered over the web: Review and analysis of key components. Patient Educ. Couns. 2009, 74, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, P.M.; Sheeran, P. Implementation intentions and goal achievement: A meta-analyses of effects and processes. Adv. Exp. Soc. Psychol. 2006, 38, 69–119. [Google Scholar]
- Bélanger-Gravel, A.; Godin, G.; Amireault, S. A meta-analytic review of the effect of implementation intentions on physical activity. Health Psychol. Rev. 2013, 7, 23–54. [Google Scholar] [CrossRef]
- Maes, S.; Karoly, P. Self-regulation assessment and intervention in physical health and illness: A review. Appl. Psychol. 2005, 54, 267–299. [Google Scholar] [CrossRef]
- Peels, D.A.; Bolman, C.; Golsteijn, R.H.J.; De Vries, H.; Mudde, A.N.; van Stralen, M.M.; Lechner, L. Differences in reach and attrition between web-based and print-delivered tailored interventions among adults over 50 years of age: Clustered randomized trial. J. Med. Int. Res. 2012, 14, e179. [Google Scholar] [CrossRef] [PubMed]
- Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F. Reach out and Touch; Maastricht University: Maastricht, Limburg, The Netherlands, 2013. [Google Scholar]
- Meyer, C.; Ulbricht, S.; Gross, B.; Kastel, L.; Wittrien, S.; Klein, G.; Skoeries, B.A.; Rumpf, H.J.; John, U. Adoption, reach and effectiveness of computer-based, practitioner delivered and combined smoking interventions in general medical practices: A three-arm cluster randomized trial. Drug Alcohol Depend. 2012, 121, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Spittaels, H.; De Bourdeaudhuij, I. Implementation of an online tailored physical activity intervention for adults in belgium. Health Promot. Int. 2006, 21, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.S.; McFarlane, M.; King, D. Barriers to std/hiv prevention on the internet. Health Educ. Res. 2001, 16, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Dickfos, M.; King, D.; Parekh, S.; Boyle, F.M.; Vandelanotte, C. General practitioners’ perceptions of and involvement in health behaviour change: Can computer-tailored interventions help? Prim. Health Care Res. Dev. 2015, 16, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Parekh, S.; King, D.; Boyle, F.M.; Vandelanotte, C. Randomized controlled trial of a computer-tailored multiple health behaviour intervention in general practice: 12-month follow-up results. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Prevost, A.T.; Wright, A.J.; Charlton, J.; Rudisill, C.; Gulliford, M.C. Effectiveness of interventions to promote healthy diet in primary care: Systematic review and meta-analysis of randomised controlled trials. BMC Public Health 2013, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ampt, A.J.; Amoroso, C.; Harris, M.F.; McKenzie, S.H.; Rose, V.K.; Taggart, J.R. Attitudes, norms and controls influencing lifestyle risk factor management in general practice. BMC Fam. Pract. 2009, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Keen, S.; Neal, R.D. Can general practitioners influence the nation’s health through a population approach to provision of lifestyle advice? Br. J. Gen. Pract. 2012, 50, 455–459. [Google Scholar]
- Carroll, J.K.; Lewis, B.A.; Marcus, B.H.; Lehman, E.B.; Shaffer, M.L.; Sciamanna, C.N. Computerized tailored physical activity reports. A randomized controlled trial. Am. J. Prev. Med. 2010, 39, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lambe, B.; Collins, C. A qualitative study of lifestyle counselling in general practice in Ireland. Fam. Pract. 2009, 27, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Brotons, C.; Bjorkelund, C.; Bulc, M.; Ciurana, R.; Godycki-Cwirko, M.; Jurgova, E.; Kloppe, P.; Lionis, C.; Mierzecki, A.; Pineiro, R.; et al. Prevention and health promotion in clinical practice: The views of general practitioners in europe. Prev. Med. 2005, 40, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Helgason, A.R.; Lund, K.E. General practitioners’ perceived barriers to smoking cessation-results from four nordic countries. Scand. J. Soc. Med. 2002, 30, 141–147. [Google Scholar]
- Vogt, F.; Hall, S.; Marteau, T.M. General practitioners’ and family physicians’ negative beliefs and attitudes towards discussing smoking cessation with patients: A systematic review. Addiction 2005, 100, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J. Promotion of physical activity in primary health care: Update of the evidence on interventions. J. Sci. Med. Sport 2004, 7, 67–73. [Google Scholar] [CrossRef]
- Sciamanna, C.N.; Novak, S.P.; Houston, T.K.; Gramling, R.; Marcus, B.H. Visit satisfaction and tailored health behaviour communications in primary care. Am. J. Prev. Med. 2004, 26, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.G.; Byers, T.E.; Christian, K.K.; Goldstein, M.G.; Bock, B.C.; Prioreschi, B.; Bessesen, D.H. A computer support program that helps clinicians provide patients with metabolic syndrome tailored counseling to promote weight loss. J. Am. Diet. Assoc. 2011, 111, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Shakeshaft, A.; Fawcett, J.; Mattick, R.P.; Richmond, R.; Wodak, A.; Harris, M.F.; Doran, C.M. Patient-driven computers in primary care: Their use and feasibility. Health Educ. 2006, 106, 400–411. [Google Scholar] [CrossRef]
- Parekh, S.; Vandelanotte, C.; King, D.; Boyle, F.M. Improving diet, physical activity and other lifestyle behaviours using computer-tailored advice in general practice: A randomised controlled trial. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Plaete, J.; De Bourdeaudhuij, I.; Verloigne, M.; Crombez, G. Acceptability, feasibility and effectiveness of an ehealth behaviour intervention using self-regulation: ‘Myplan’. Patient Educ. Couns. 2015, 98, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Spittaels, H.; De Bourdeaudhuij, I.; Brug, J.; Vandelanotte, C. Effectiveness of an online computer-tailored physical activity intervention in a real-life setting. Health Educ. Res. 2007, 22, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, R. Modeling health behaviour change: How to predict and modify the adoption and maintenance of health behaviours. Appl. Psychol. 2008, 57, 1–29. [Google Scholar]
- Plaete, J.; De Bourdeaudhuij, I.; Verloigne, M.; Oenema, A.; Crombez, G. A self-regulation ehealth intervention to increase healthy behaviour through general practice: Protocol and systematic development. JMIR Res. Protoc. 2015, 4, e141. [Google Scholar] [CrossRef] [PubMed]
- Glynn, L.G.; Hayes, P.S.; Casey, M.; Glynn, F.; Alvarez-Iglesias, A.; Newell, J.; ÓLaighin, G.; Heaney, D.; O’Donnell, M.; Murphy, A.W. Effectiveness of a smartphone application to promote physical activity in primary care: The smart move randomised controlled trial. Br. J. Gen. Pract. 2014, 64, e384–e391. [Google Scholar] [CrossRef] [PubMed]
- Van Keulen, H.M.; Mesters, I.; Ausems, M.; van Breukelen, G.; Campbell, M.; Resnicow, K.; Brug, J.; de Vries, H. Tailored print communication and telephone motivational interviewing are equally successful in improving multiple lifestyle behaviours in a randomized controlled trial. Ann. Behav. Med. 2011, 41, 104–118. [Google Scholar] [CrossRef] [PubMed]
- De Cocker, K.; Spittaels, H.; Cardon, G.; De Bourdeaudhuij, I.; Vandelanotte, C. Web-based, computer-tailored, pedometer-based physical activity advice: Development, dissemination through general practice, acceptability, and preliminary efficacy in a randomized controlled trial. J. Med. Internet Res. 2012, 14, e53. [Google Scholar] [CrossRef] [PubMed]
- Sniehotta, F.F.; Scholz, U.; Schwarzer, R. Bridging the intention–behaviour gap: Planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychol. Health 2005, 20, 143–160. [Google Scholar] [CrossRef]
- Conn, V.S.; Hafdahl, A.R.; Mehr, D.R. Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. Am. J. Public Health 2011, 101, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Bully, P.; Martinez, C.; Grandes, G. Effectiveness of physical activity promotion interventions in primary care: A review of reviews. Prev. Med. 2015, 76, S56–S67. [Google Scholar] [CrossRef] [PubMed]
- Middelweerd, A.; Mollee, J.S.; van der Wal, C.N.; Brug, J.; Te Velde, S.J. Apps to promote physical activity among adults: A review and content analysis. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Compernolle, S.; Vandelanotte, C.; Cardon, G.; De Bourdeaudhuij, I.; De Cocker, K. Effectiveness of a web-based, computer-tailored, pedometer-based physical activity intervention for adults: A cluster randomized controlled trial. J. Med. Internet Res. 2015, 17, e38. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.; Schoeppe, S.; Guertler, D. Interest and preferences for using advanced physical activity tracking devices: Results of a national cross-sectional survey. BMJ Open 2016, 6, e011243. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J.; Gupta, S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Stadler, G.; Oettingen, G.; Gollwitzer, P.M. Physical activity in women: Effects of a self-regulation intervention. Am. J. Prev. Med. 2009, 36, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.S.; Wojcik, J.R.; Winett, R.A.; Williams, D.M. Social-cognitive determinants of physical activity: The influence of social support, self-efficacy, outcome expectations, and self-regulation among participants in a church-based health promotion study. Health Psychol. 2006, 25, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Van der Mispel, C.; Poppe, L.; Crombez, G.; Verloigne, M.; De Bourdeaudhuij, I. A self-regulation-based eHealth intervention to promote a healthy lifestyle: Investigating user and website characteristics related to attrition. J. Med. Internet Res. 2017, 19, e241. [Google Scholar] [CrossRef] [PubMed]
- Sallis, J.F.; Saelens, B.E. Assessment of physical activity by self-report: Status, limitations, and future directions. Res. Q. Exerc. Sport 2000, 71 (Suppl. 2), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Krebs, P.; Prochaska, J.O.; Rossi, J.S. A meta-analysis of computer-tailored interventions for health behaviour change. Prev. Med. 2010, 51, 214–221. [Google Scholar] [CrossRef] [PubMed]
| Intervention Group | Wait-List Control Group | |||
|---|---|---|---|---|
| Receive flyer with instructions to participate in the study | Receive flyer with instructions to fill out the evaluation questionnaire | |||
| T0 | Sign in on a tablet in general practice | Sign in on the website (e.g., back at home) | Sign in on a tablet in general practice | Sign in on the website (e.g., back at home) |
| Selection of PA as target behavior Fill out IPAQ Receive tailored feedback Use the self-regulation tool (personal plan with action and coping plan) Receive email with personal plan | Fill out IPAQ Receive general feedback | |||
| Receive invitation email | Receive invitation email | |||
| T1 | Fill out IPAQ Receive tailored feedback on the behaviour change process Maintain or adapt personal plan Receive email with personal plan | Fill out IPAQ | ||
| Receive invitation email | Receive invitation email | |||
| T2 | Fill out IPAQ Receive tailored feedback on the behaviour change process Maintain or adapt personal plan Email with personal plan | Fill out IPAQ | ||
| Intervention Group | Control Group | |
|---|---|---|
| (n = 94) | (n = 118) | |
| Age (mean ± SD years) | 43.51 ± 14.70 | 46.14 ± 14.76 |
| Gender (% male) | 27.8 | 33.1 |
| Education level (% high = university or college degree) | 48.9 | 49.2 |
| BMI (mean ± SD kg/m2) | 25.56 ± 5.24 | 25.21 ± 5.10 |
| Not meeting MVPA recommendations (%) | 42.2 | 33.9 |
| PA outcomes (mean ± SD min/week) Total PA | 683.39 ± 500.89 | 733.96 ± 548.36 |
| Vigorous PA | 88.30 ± 144.93 | 119.13 ± 1778.66 |
| MVPA | 395.95 ± 383.63 | 444.41 ± 430.31 |
| Total PA | Vigorous PA | MVPA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Null Model 1 | Model 1a | Model 1b | Null Model 2 | Model 2a | Model 2b | Null Model 3 | Model 3a | Model 3b |
| Fixed Part | β (S.E.) | β (S.E.) | β (S.E.) | β (S.E.) | β (S.E.) | β (S.E.) | β (S.E.) | β (S.E.) | β (S.E.) |
| Intercept | 706.83 (35.76) | 860.42 (55.24) | 903.76 (65.97) | 106.80 (10.94) | 136.03 (0.74) | 113.42 (21.19) | 418.51(35.80) | 475.22 (46.56) | 518.00 |
| Age | −2.92 (2.50) | −3.04 (2.51) | −3.54 (0.74) *** | −3.65 (0.74) *** | −5.88 (1.84) ** | −5.98 (1.86) ** | |||
| Gender | −114.23 (77.00) | 115.94 (77.11) | 29.80 (22.66) | 27.60 (22.60) | 62.35 (55.69) | 60.21 (55.78) | |||
| Education | 249.38 (70.12) ** | 24.44 (70.10) | 77.09 (20.79) ** | 77.20 (20.65) ** | −163.50 (52.25) ** | −163.54 (52.21) ** | |||
| BMI | 0.77 (7.10) | 0.97 (7.12) | 1.15 (2.10) | 1.35 (2.09) | 1.92 (5.26) | 2.19 (5.28) | |||
| Condition | −78.91 (75.48) | 42.22 (22.74) * | −64.62 (59.78) | ||||||
| Time | −62.14 (33.29) | 12.22 (12.87) | −57.10 (24.31) ** | ||||||
| Time * group | 103.38 (50.73) ** | 24.07 (17.06) | 74.97 (37.04) ** | ||||||
| Random Part | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) | σ2 (S.E.) |
| Time-level variance | 68,746.27 (6741.13) *** | 65,198.83 (6487.53) *** | 63,733.49 (6341.72) *** | 7963.66 (780.901) *** | 7282.02 (724.59) *** | 7209.91 (717.41) *** | 36,057.49 (3384.52) *** | 34,889.68 (3326.60) *** | 33,968.72 (3238.79) *** |
| GP-level variance | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 251.51 (723.52) | 166.69 (689.42) | 11,231.87 (7735.48) | 9516.69 (7009.75) | 8949.41 (6832.96) |
| Adult-level variance | 231,589.33 (26,296.67 ) *** | 212,810.52 (24,633.71) *** | 213,365.22 (24,606.65) *** | 20,896.51 (2471.57) *** | 17,457.61 (2224.60) *** | 17,357.36 (2209.51) *** | 127,582.45 (3384.52) *** | 118,342.31 (13,611.74) *** | 118,918.18 (13,614.77) *** |
| Deviance test model | 6239.62 | 6032.69 | 6027.95 | 5298.45 | 5096.50 | 5092.459 | 6538.46 | 6313.52 | 6307.321 |
| χ2 (df) | 206.93 (4) *** | 211.67 (7) *** | 201.95 (4) *** | 205.99 (7) *** | 224.94 (4) *** | 231.14 (7) *** | |||
| Intervention Group (S.E.) | Wait-List Control Group (S.E.) | ||
|---|---|---|---|
| Total PA (min/week) | Pre | 683.39 (57.70) | 733.95 (50.39) |
| Post | 731.43 (57.70) | 678.82 (50.39) | |
| Vigorous PA (min/week) | Pre | 404.79 (47.59) | 445.10 (42.38) |
| Post | 426.64 (47.59) | 391.42 (42.38) | |
| MVPA (min/week) | Pre | 88.30 (17.87) | 119.13 (15.61) |
| Post | 105.98 (17.87) | 109.21 (15.61) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degroote, L.; Plaete, J.; De Bourdeaudhuij, I.; Verloigne, M.; Van Stappen, V.; De Meester, A.; Poppe, L.; Van der Mispel, C.; Crombez, G. The Effect of the eHealth Intervention ‘MyPlan 1.0’ on Physical Activity in Adults Who Visit General Practice: A Quasi-Experimental Trial. Int. J. Environ. Res. Public Health 2018, 15, 228. https://doi.org/10.3390/ijerph15020228
Degroote L, Plaete J, De Bourdeaudhuij I, Verloigne M, Van Stappen V, De Meester A, Poppe L, Van der Mispel C, Crombez G. The Effect of the eHealth Intervention ‘MyPlan 1.0’ on Physical Activity in Adults Who Visit General Practice: A Quasi-Experimental Trial. International Journal of Environmental Research and Public Health. 2018; 15(2):228. https://doi.org/10.3390/ijerph15020228
Chicago/Turabian StyleDegroote, Laurent, Jolien Plaete, Ilse De Bourdeaudhuij, Maïté Verloigne, Vicky Van Stappen, An De Meester, Louise Poppe, Celien Van der Mispel, and Geert Crombez. 2018. "The Effect of the eHealth Intervention ‘MyPlan 1.0’ on Physical Activity in Adults Who Visit General Practice: A Quasi-Experimental Trial" International Journal of Environmental Research and Public Health 15, no. 2: 228. https://doi.org/10.3390/ijerph15020228
APA StyleDegroote, L., Plaete, J., De Bourdeaudhuij, I., Verloigne, M., Van Stappen, V., De Meester, A., Poppe, L., Van der Mispel, C., & Crombez, G. (2018). The Effect of the eHealth Intervention ‘MyPlan 1.0’ on Physical Activity in Adults Who Visit General Practice: A Quasi-Experimental Trial. International Journal of Environmental Research and Public Health, 15(2), 228. https://doi.org/10.3390/ijerph15020228





