Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy
Abstract
:1. Introduction
- compare the distribution of u(iAs+MMA+DMA) with other baseline international studies;
- assess the relationship between the u(iAs+MMA+DMA) concentration and various exposure factors investigated by the HBM questionnaire using stepwise multiple regression.
2. Materials and Methods
- usual consumption (more than twice a week) of tap water for drinking or cooking;
- usual consumption (more than twice a week) of meat, seafood, vegetables and fruit, bread, whole milk, coffee, wine, and beer;
- usual consumption (more than twice a week) of locally-produced meat, seafood, vegetables or fruit, bread and whole milk;
- occupational exposure in chemical industries and to industrial dust, chemical substances, gases, in particular: exposure to inorganic solvents and acids, oil derivatives, and silica (As is used for doping microchips);
- active cigarette smokers or former smokers who had given up less than six months ago;
- seafood consumption during three days before urine collection.
3. Results
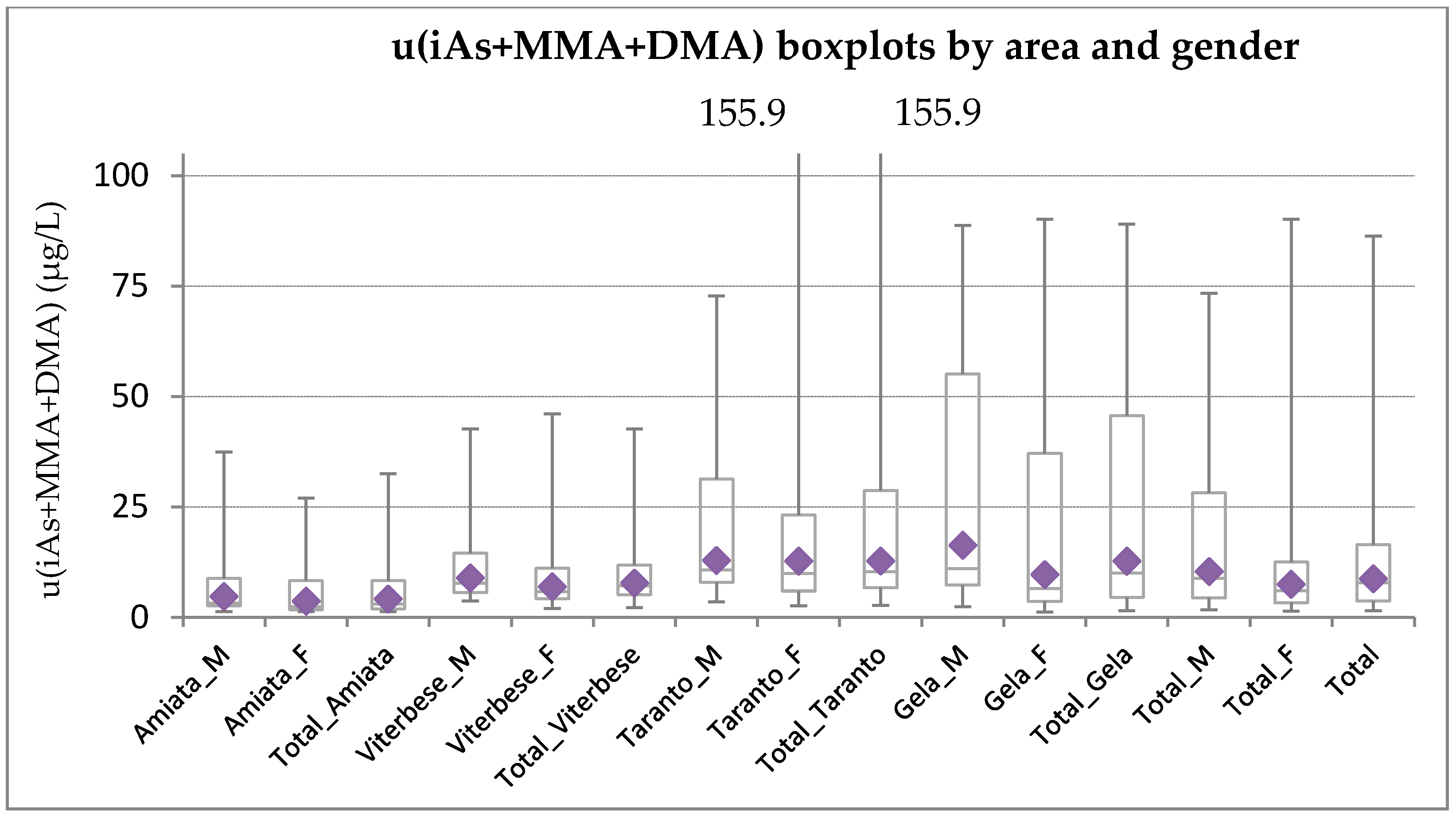
3.1. Distribution of u(iAs+uMMA+uDMA) Levels by Area and Gender
3.2. Stepwise Multiple Regression
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ATSDR-Toxicological Profile: Arsenic. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3 (accessed on 18 December 2017).
- Straif, K.; Benbrahim-Tallaa, L.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part C: Metals, arsenic, dusts, and fibres. Lancet Oncol. 2009, 10, 453–454. [Google Scholar] [CrossRef]
- IARC Monographs-Monographs Available in PDF Format. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100C/index.php (accessed on 18 December 2017).
- Navas-Acien, A.; Sharrett, A.R.; Silbergeld, E.K.; Schwartz, B.S.; Nachman, K.E.; Burke, T.A.; Guallar, E. Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. Am. J. Epidemiol. 2005, 162, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Silbergeld, E.K.; Streeter, R.A.; Clark, J.M.; Burke, T.A.; Guallar, E. Arsenic exposure and type 2 diabetes: A systematic review of the experimental and epidemiological evidence. Environ. Health Perspect. 2006, 114, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Silbergeld, E.K.; Pastor-Barriuso, R.; Guallar, E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008, 300, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rossman, T.G. Effect of metals on mutagenesis and DNA repair. Environ. Health Perspect. 1981, 40, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, A.D.; Doerr, C.L.; Tennant, A.H.; Harrington-Brock, K.; Allen, J.W.; Winkfield, E.; Poorman-Allen, P.; Kundu, B.; Funasaka, K.; Roop, B.C.; et al. Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic: Induction of chromosomal mutations but not gene mutations. Environ. Mol. Mutagen. 2003, 42, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.B.; Leszczynska, J.; Hickey, C.; Rossman, T.G. Further evidence against a direct genotoxic mode of action for arsenic-induced cancer. Toxicol. Appl. Pharmacol. 2007, 222, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Ma, H.; Feng, Z.; Xia, Y.; Le, X.C.; Ni, Z.; Allen, J.; Collins, B.; Schreinemachers, D.; Mumford, J.L. Analyses of micronuclei in exfoliated epithelial cells from individuals chronically exposed to arsenic via drinking water in inner Mongolia, China. J. Toxicol. Environ. Health Part A 2001, 64, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.-H.; Chen, Y.-H.; Loh, C.-H.; Yang, T.; Wu, T.-N.; Chen, C.-J.; Hsieh, L.-L. The association between frequencies of mitomycin C-induced sister chromatid exchange and cancer risk in arseniasis. Toxicol. Lett. 2002, 129, 237–243. [Google Scholar] [CrossRef]
- Basu, A.; Mahata, J.; Roy, A.K.; Sarkar, J.N.; Poddar, G.; Nandy, A.K.; Sarkar, P.K.; Dutta, P.K.; Banerjee, A.; Das, M.; et al. Enhanced frequency of micronuclei in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2002, 516, 29–40. [Google Scholar] [CrossRef]
- Basu, A.; Ghosh, P.; Das, J.K.; Banerjee, A.; Ray, K.; Giri, A.K. Micronuclei as biomarkers of carcinogen exposure in populations exposed to arsenic through drinking water in West Bengal, India: A comparative study in three cell types. Cancer Epidemiol. Prev. Biomark. 2004, 13, 820–827. [Google Scholar]
- Mahata, J.; Basu, A.; Ghoshal, S.; Sarkar, J.N.; Roy, A.K.; Poddar, G.; Nandy, A.K.; Banerjee, A.; Ray, K.; Natarajan, A.T.; et al. Chromosomal aberrations and sister chromatid exchanges in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat. Res. 2003, 534, 133–143. [Google Scholar] [CrossRef]
- Mahata, J.; Chaki, M.; Ghosh, P.; Das, L.K.; Baidya, K.; Ray, K.; Natarajan, A.T.; Giri, A.K. Chromosomal aberrations in arsenic-exposed human populations: A review with special reference to a comprehensive study in West Bengal, India. Cytogenet. Genome Res. 2004, 104, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Creus, A.; Venegas, W.; Arroyo, A.; Beck, J.P.; Gebel, T.W.; Surrallés, J.; Marcos, R. Evaluation of micronucleus induction in a Chilean population environmentally exposed to arsenic. Mutat. Res. 2004, 564, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Creus, A.; Venegas, W.; Arroyo, A.; Beck, J.P.; Gebel, T.W.; Surrallés, J.; Marcos, R. Micronuclei assessment in buccal cells of people environmentally exposed to arsenic in northern Chile. Toxicol. Lett. 2005, 155, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Das, U.; Poddar, S.; Sengupta, B.; De, M. Micronuclei and chromosomal aberrations as biomarkers: A study in an arsenic exposed population in West Bengal, India. Bull. Environ. Contam. Toxicol. 2006, 76, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Basu, A.; Mahata, J.; Basu, S.; Sengupta, M.; Das, J.K.; Mukherjee, A.; Sarkar, A.K.; Mondal, L.; Ray, K.; et al. Cytogenetic damage and genetic variants in the individuals susceptible to arsenic-induced cancer through drinking water. Int. J. Cancer 2006, 118, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Banerjee, M.; De Chaudhuri, S.; Das, J.K.; Sarma, N.; Basu, A.; Giri, A.K. Increased chromosome aberration frequencies in the Bowen’s patients compared to non-cancerous skin lesions individuals exposed to arsenic. Mutat. Res. 2007, 632, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Sarma, N.; Biswas, R.; Roy, J.; Mukherjee, A.; Giri, A.K. DNA repair deficiency leads to susceptibility to develop arsenic-induced premalignant skin lesions. Int. J. Cancer 2008, 123, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Sampayo-Reyes, A.; Hernández, A.; El-Yamani, N.; López-Campos, C.; Mayet-Machado, E.; Rincón-Castañeda, C.B.; de Limones-Aguilar, M.L.; López-Campos, J.E.; de León, M.B.; González-Hernández, S.; et al. Arsenic induces DNA damage in environmentally exposed Mexican children and adults. Influence of GSTO1 and AS3MT polymorphisms. Toxicol. Sci. 2010, 117, 63–71. [Google Scholar] [CrossRef] [PubMed]
- WHO. Arsenic. Available online: http://www.who.int/ipcs/assessment/public_health/arsenic/en/ (accessed on 18 December 2017).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Arsenic in Food: Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; Turco, A.C.; Mantovani, A. Speciated urinary arsenic as a biomarker of dietary exposure to inorganic arsenic in residents living in high-arsenic areas in Latium, Italy. Pure Appl. Chem. 2012, 84, 203–214. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M. Mechanisms of arsenic biotransformation. Toxicology 2002, 181–182, 211–217. [Google Scholar] [CrossRef]
- Dopp, E.; Hartmann, L.M.; von Recklinghausen, U.; Florea, A.M.; Rabieh, S.; Zimmermann, U.; Shokouhi, B.; Yadav, S.; Hirner, A.V.; Rettenmeier, A.W. Forced uptake of trivalent and pentavalent methylated and inorganic arsenic and its cyto-/genotoxicity in fibroblasts and hepatoma cells. Toxicol. Sci. 2005, 87, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yu, J.; Li, H.; Yang, L.; Xia, Y.; Wu, K.; Gao, J.; Guo, Z.; Cui, N. Arsenic Metabolites and Methylation Capacity Among Individuals Living in a Rural Area with Endemic Arseniasis in Inner Mongolia, China. Biol. Trace Elem. Res. 2016, 170, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Drobna, Z.; Styblo, M.; Thomas, D.J. An Overview of Arsenic Metabolism and Toxicity. Curr. Protoc. Toxicol. 2009, 42. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, K.T. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001, 172, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Stýblo, M.; Drobná, Z.; Jaspers, I.; Lin, S.; Thomas, D.J. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Environ. Health Perspect. 2002, 110, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci. Prog. 1999, 82, 69–88. [Google Scholar] [PubMed]
- Vahter, M. Variation in Human Metabolism of Arsenic. In Arsenic Exposure and Health Effects III; Chappell, W.R., Abernathy, C.O., Calderon, R.L., Eds.; Elsevier Science Ltd.: Oxford, UK, 1999; pp. 267–279. ISBN 978-0-08-043648-7. [Google Scholar]
- Tseng, C.-H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol. 2009, 235, 338–350. [Google Scholar] [CrossRef] [PubMed]
- WHO. Environmental Health Criteria 224: Arsenic and Arsenic Compounds. Available online: http://www.who.int/ipcs/publications/ehc/ehc_224/en/ (accessed on 18 December 2017).
- Bustaffa, E.; Bianchi, F. Studies on markers of exposure and early effect in areas with arsenic pollution: Methods and results of the project SEpiAs. Epidemiological studies on population exposed to low-to-moderate arsenic concentration in drinking water. Epidemiol. Prev. 2014, 38, 14–24. [Google Scholar] [PubMed]
- Smith, A.H.; Goycolea, M.; Haque, R.; Biggs, M.L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 1998, 147, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Chen, Y.; Parvez, F.; Zablotska, L.; Argos, M.; Hussain, I.; Momotaj, H.; Levy, D.; Cheng, Z.; Slavkovich, V.; et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: Baseline results from the Health Effects of Arsenic Longitudinal Study. Am. J. Epidemiol. 2006, 163, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.E.; Li, L.; Nermell, B.; Rahman, A.; El Arifeen, S.; Rahman, M.; Persson, L.A.; Ekström, E.-C. Arsenic exposure in pregnancy: A population-based study in Matlab, Bangladesh. J. Health Popul. Nutr. 2006, 24, 236–245. [Google Scholar] [PubMed]
- Chen, Y.; Graziano, J.H.; Parvez, F.; Liu, M.; Slavkovich, V.; Kalra, T.; Argos, M.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 2011, 342, d2431. [Google Scholar] [CrossRef] [PubMed]
- Dauphiné, D.C.; Smith, A.H.; Yuan, Y.; Balmes, J.R.; Bates, M.N.; Steinmaus, C. Case-control study of arsenic in drinking water and lung cancer in California and Nevada. Int. J. Environ. Res. Public Health 2013, 10, 3310–3324. [Google Scholar] [CrossRef] [PubMed]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Monrad, M.; Ersbøll, A.K.; Sørensen, M.; Baastrup, R.; Hansen, B.; Gammelmark, A.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level arsenic in drinking water and risk of incident myocardial infarction: A cohort study. Environ. Res. 2017, 154, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Cubadda, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Aureli, F.; Carcea, M. Arsenic contamination of the environment-food chain: A survey on wheat as a test plant to investigate phytoavailable arsenic in Italian agricultural soils and as a source of inorganic arsenic in the diet. J. Agric. Food Chem. 2010, 58, 10176–10183. [Google Scholar] [CrossRef] [PubMed]
- Cubadda, F.; D’Amato, M.; Mancini, F.R.; Aureli, F.; Raggi, A.; Busani, L.; Mantovani, A. Assessing human exposure to inorganic arsenic in high-arsenic areas of Latium: A biomonitoring study integrated with indicators of dietary intake. Ann. Ig. 2015, 27, 39–51. [Google Scholar] [PubMed]
- Bustaffa, E.; Minichilli, F.; Andreassi, M.G.; Carone, S.; Coi, A.; Cori, L.; Faita, F.; Faita, F.; Grecchi, S.; Minoia, C.; et al. Studies on markers of exposure and early effect in areas with arsenic pollution: Methods and results of the project SEpiAs. Epidemiological surveillance in areas with environmental pollution by natural or anthropogenic arsenic. Epidemiol. Prev. 2014, 38, 27–94. [Google Scholar] [PubMed]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0032:0054:EN:PDF (accessed on 18 December 2017).
- Scientific Committee on Health and Environmental Risks (SCHER). Derogation on the Drinking Water Directive 98/83/EC. 2010. Available online: https://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_120.pdf (accessed on 18 December 2017).
- Minichilli, F.; Nuvolone, D.; Bustaffa, E.; Cipriani, F.; Vigotti, M.A.; Bianchi, F. State of health of populations residing in geothermal areas of Tuscany. Epidemiol. Prev. 2012, 36, 1–104. [Google Scholar] [PubMed]
- Musmeci, L.; Bianchi, F.; Carere, M.; Cori, L. Environment and health in Gela (Sicily): Present knowledge and prospects for future studies. Epidemiol. Prev. 2009, 33, 7–12. [Google Scholar] [PubMed]
- Musmeci, L.; Carere, M.; Fallenti, F. Environmental pollution in the area of Gela. Epidemiol. Prev. 2009, 33, 18–23. [Google Scholar] [PubMed]
- Comba, P.; Pirastu, R.; Conti, S.; De Santis, M.; Iavarone, I.; Marsili, G.; Mincuzzi, A.; Minelli, G.; Manno, V.; Minerba, S.; et al. Environment and health in Taranto, southern Italy: Epidemiological studies and public health recommendations. Epidemiol. Prev. 2012, 36, 305–320. [Google Scholar] [PubMed]
- Eurachem-The fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics (2014). Available online: https://www.eurachem.org/index.php/publications/guides/mv (accessed on 30 January 2018).
- CDC-National Report on Human Exposure to Environmental Chemicals-NER. Available online: https://www.cdc.gov/exposurereport/index.html (accessed on 18 December 2017).
- Schläwicke Engström, K.; Nermell, B.; Concha, G.; Strömberg, U.; Vahter, M.; Broberg, K. Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 667, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-H.; Sung, B.H.; Kim, S.-S.; Rhim, H.; Kuh, H.-J. Synergistic interaction between tetra-arsenic oxide and paclitaxel in human cancer cells in vitro. Int. J. Oncol. 2009, 34, 1669–1679. [Google Scholar] [PubMed]
- Valenzuela, O.L.; Drobná, Z.; Hernández-Castellanos, E.; Sánchez-Peña, L.C.; García-Vargas, G.G.; Borja-Aburto, V.H.; Stýblo, M.; Del Razo, L.M. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol. Appl. Pharmacol. 2009, 239, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Xu, Y.; Wang, Y.; Jin, Y.; Itoh, T.; Yoshida, T.; Sun, G. Arsenic methylation capacity and its correlation with skin lesions induced by contaminated drinking water consumption in residents of chronic arsenicosis area. Environ. Toxicol. 2011, 26, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Crecelius, E.A. Changes in the chemical speciation of arsenic following ingestion by man. Environ. Health Perspect. 1977, 19, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Liu, J.; Wang, D.; Zheng, Q.; Sun, G. Differences of urinary arsenic metabolites and methylation capacity between individuals with and without skin lesions in Inner Mongolia, Northern China. Int. J. Environ. Res. Public Health 2014, 11, 7319–7332. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M.V.; Liu, X.; Ahsan, H.; Pilsner, R.; Ilievski, V.; Slavkovich, V.; Parvez, F.; Levy, D.; Factor-Litvak, P.; Graziano, J.H. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ. Health Perspect. 2005, 113, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Brima, E.I.; Haris, P.I.; Jenkins, R.O.; Polya, D.A.; Gault, A.G.; Harrington, C.F. Understanding arsenic metabolism through a comparative study of arsenic levels in the urine, hair and fingernails of healthy volunteers from three unexposed ethnic groups in the United Kingdom. Toxicol. Appl. Pharmacol. 2006, 216, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Chen, Y.; Kibriya, M.G.; Slavkovich, V.; Parvez, F.; Jasmine, F.; Gamble, M.V.; Graziano, J.H. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Prev. Biomark. 2007, 16, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Yuan, Y.; Liaw, J.; Smith, A.H. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: A reanalysis. Epidemiology 2009, 20, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.; Edmonds, J.; Francesconi, K.; Raston, C.; Saunders, J.; Skelton, B.; White, A. Isolation, crystal structure and synthesis of arsenobetaine, a constituent of the western rock lobster, the dusky shark, and some samples of human urine. Aust. J. Chem. 1981, 34, 787–798. [Google Scholar] [CrossRef]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Edmonds, J.S. Arsenic and Marine Organisms. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 44, pp. 147–189. [Google Scholar]
- Brown, R.M.; Newton, D.; Pickford, C.J.; Sherlock, J.C. Human metabolism of arsenobetaine ingested with fish. Hum. Exp. Toxicol. 1990, 9, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Le, X.C.; Cullen, W.R.; Reimer, K.J. Human urinary arsenic excretion after one-time ingestion of seaweed, crab, and shrimp. Clin. Chem. 1994, 40, 617–624. [Google Scholar] [PubMed]
- Bild, D.E.; Bluemke, D.A.; Burke, G.L.; Detrano, R.; Diez Roux, A.V.; Folsom, A.R.; Greenland, P.; Jacob, D.R.; Kronmal, R.; Liu, K.; et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am. J. Epidemiol. 2002, 156, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, K.A.; Tanggaar, R.; McKenzie, C.J.; Goessler, W. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin. Chem. 2002, 48, 92–101. [Google Scholar] [PubMed]
- Jones, M.R.; Tellez-Plaza, M.; Vaidya, D.; Grau, M.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Post, W.S.; Kaufman, J.D.; Navas-Acien, A. Estimation of Inorganic Arsenic Exposure in Populations with Frequent Seafood Intake: Evidence From MESA and NHANES. Am. J. Epidemiol. 2016, 184, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-S.; Choi, S.-J.; Kim, D.-W.; Huang, M.; Kim, N.-Y.; Park, K.-S.; Kim, C.-Y.; Lee, H.-M.; Yum, Y.-N.; Han, E.-S.; et al. Effects of repeated seafood consumption on urinary excretion of arsenic species by volunteers. Arch. Environ. Contam. Toxicol. 2010, 58, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn-Rich, C.; Biggs, M.L.; Smith, A.H.; Kalman, D.A.; Moore, L.E. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ. Health Perspect. 1996, 104, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Umans, J.G.; Howard, B.V.; Goessler, W.; Francesconi, K.A.; Crainiceanu, C.M.; Silbergeld, E.K.; Guallar, E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: The Strong Heart Study. Environ. Health Perspect. 2009, 117, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Francesconi, K.A.; Silbergeld, E.K.; Guallar, E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ. Res. 2011, 111, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, B.E.; Knutsen, H.K.; Haugen, M.; Gjelstad, I.M.; Jenssen, M.T.S.; Ellingsen, D.G.; Thomassen, Y.; Alexander, J.; Meltzer, H.M.; Brantsæter, A.L. Essential and toxic element concentrations in blood and urine and their associations with diet: Results from a Norwegian population study including high-consumers of seafood and game. Sci. Total Environ. 2013, 463–464, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Hakala, E.; Pyy, L. Assessment of exposure to inorganic arsenic by determining the arsenic species excreted in urine. Toxicol. Lett. 1995, 77, 249–258. [Google Scholar] [CrossRef]
- Hata, A.; Endo, Y.; Nakajima, Y.; Ikebe, M.; Ogawa, M.; Fujitani, N.; Endo, G. HPLC-ICP-MS speciation analysis of arsenic in urine of Japanese subjects without occupational exposure. J. Occup. Health 2007, 49, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, A.; Zeghnoun, A.; Bidondo, M.-L.; Garnier, R.; Cirimele, V.; Persoons, R.; Fréry, N. Urinary arsenic levels in the French adult population: The French National Nutrition and Health Study, 2006–2007. Sci. Total Environ. 2012, 433, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.L.; Hudgens, E.E.; Carty, C.; He, B.; Le, X.C.; Rogers, J.; Thomas, D.J. Biological and behavioral factors modify biomarkers of arsenic exposure in a U.S. population. Environ. Res. 2013, 126, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Mansilla-Rivera, I.; Nazario, C.M.; Ramírez-Marrero, F.A.; Crespo, C.J.; Rodríguez-Sierra, C.J. Assessing arsenic exposure from consumption of seafood from Vieques-Puerto Rico: A pilot biomonitoring study using different biomarkers. Arch. Environ. Contam. Toxicol. 2014, 66, 162–175. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 13; StataCorp. LP: College Station, TX, USA, 2013. [Google Scholar]
- Janasik, B.; Reszka, E.; Stanislawska, M.; Wieczorek, E.; Fendler, W.; Wasowicz, W. Biological monitoring and the influence of genetic polymorphism of As3MT and GSTs on distribution of urinary arsenic species in occupational exposure workers. Int. Arch. Occup. Environ. Health 2015, 88, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Offergelt, J.A.; Roels, H.; Buchet, J.P.; Boeckx, M.; Lauwerys, R. Relation between airborne arsenic trioxide and urinary excretion of inorganic arsenic and its methylated metabolites. Occup. Environ. Med. 1992, 49, 387–393. [Google Scholar] [CrossRef]
- Valori di Riferimento-Pubblico. Available online: http://associazione.squarespace.com/pubblico/ (accessed on 18 December 2017).
- Becker, K.; Schulz, C.; Kaus, S.; Seiwert, M.; Seifert, B. German Environmental Survey 1998 (GerES III): Environmental pollutants in the urine of the German population. Int. J. Hyg. Environ. Health 2003, 206, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Conrad, A.; Becker, K.; Kolossa-Gehring, M.; Seiwert, M.; Seifert, B. Twenty years of the German Environmental Survey (GerES): Human biomonitoring—Temporal and spatial (West Germany/East Germany) differences in population exposure. Int. J. Hyg. Environ. Health 2007, 210, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Fréry, N.; Saoudi, A.; Garnier, R.; Zeghnoun, A.; Falq, G. Exposition de la Population Française Aux Substances Chimiques de L’environnement. Tome 1: Présentation Générale de L’étude Métaux et Métalloïdes. Institute de Veille Sanitaire. Available online: http://opac.invs.sante.fr/doc_num.php?explnum_id=6864 (accessed on 18 December 2017).
- Meza, M.M.; Kopplin, M.J.; Burgess, J.L.; Gandolfi, A.J. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ. Res. 2004, 96, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Baldassarre, A.; Gatti, M.F.; Gagliardi, T.; Serinelli, M.; De Maria, L.; Caputi, A.; Dirodi, A.A.; Galise, I.; Cuccaro, F.; et al. Non-occupational exposure to heavy metals of the residents of an industrial area and biomonitoring. Environ. Monit. Assess. 2016, 188, 673. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chai, Y.; Yu, J.; Wei, B.; Xia, Y.; Wu, K.; Gao, J.; Guo, Z.; Cui, N. Associations of arsenic metabolites, methylation capacity, and skin lesions caused by chronic exposure to high arsenic in tube well water. Environ. Toxicol. 2017, 32, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Carrus, A.; Sciannamblo, G.; Caputo, F.; Minunni, V.; de Nichilo, G.; Bellotta, M.R.; Gagliardi, T.; Bisceglia, L.; Assennato, G. A study of factors influencing urinary arsenic excretion in exposed workers. Int. J. Environ. Health Res. 2009, 19, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kile, M.L.; Houseman, E.A.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Hsueh, Y.-M.; Christiani, D.C. Influence of GSTT1 Genetic Polymorphisms on Arsenic Metabolism. J. Indian Soc. Agric. Stat. 2013, 67, 197–207. [Google Scholar] [PubMed]
- Fillol, C.; Dor, F.; Labat, L.; Boltz, P.; Le Bouard, J.; Mantey, K.; Mannschott, C.; Puskarczyk, E.; Viller, F.; Momas, I.; et al. Urinary arsenic concentrations and speciation in residents living in an area with naturally contaminated soils. Sci. Total Environ. 2010, 408, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.-S.; Ryu, D.-Y.; Choi, B.-S.; Park, J.-D. Urinary Arsenic Concentrations and their Associated Factors in Korean Adults. Toxicol. Res. 2013, 29, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.D.; Werlinger, F.; Orellana, M.; Jara, M.; Rocha, R.; Alvarado, S.A.; Luis, Q. Polymorphism of glutathione S-transferase (GST) variants and its effect on distribution of urinary arsenic species in people exposed to low inorganic arsenic in tap water: An exploratory study. Arch. Environ. Occup. Health 2010, 65, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Agusa, T.; Iwata, H.; Fujihara, J.; Kunito, T.; Takeshita, H.; Minh, T.B.; Trang, P.T.K.; Viet, P.H.; Tanabe, S. Genetic polymorphisms in glutathione S-transferase (GST) superfamily and arsenic metabolism in residents of the Red River Delta, Vietnam. Toxicol. Appl. Pharmacol. 2010, 242, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.A.; Smith, A.H.; Tokar, E.J.; Graziano, J.H.; Kim, K.-W.; Navasumrit, P.; Ruchirawat, M.; Thiantanawat, A.; Suk, W.A.; Fry, R.C. Mechanisms Underlying Latent Disease Risk Associated with Early-Life Arsenic Exposure: Current Research Trends and Scientific Gaps. Environ. Health Perspect. 2016, 124, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Yuan, Y.; Liaw, J.; Durán, V.; Cuevas, S.; García, J.; Meza, R.; Valdés, R.; et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, R.; Shao, K.; Thomas, D.J.; Sams, R.; Cowden, J. AS3MT, GSTO, and PNP polymorphisms: Impact on arsenic methylation and implications for disease susceptibility. Environ. Res. 2014, 132, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.A.; Wu, M.C.; Ward, W.O.; Smeester, L.; Rager, J.E.; García-Vargas, G.; Del Razo, L.-M.; Drobná, Z.; Stýblo, M.; Fry, R.C. Arsenic and the epigenome: Interindividual differences in arsenic metabolism related to distinct patterns of DNA methylation. J. Biochem. Mol. Toxicol. 2013, 27, 106–115. [Google Scholar] [CrossRef] [PubMed]
| Area | Male | Female | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–29 (%) | 30–39 (%) | 40–44 (%) | Total (%) | 20–29 (%) | 30–39 (%) | 40–44 (%) | Total (%) | 20–29 (%) | 30–39 (%) | 40–44 (%) | Total (%) | |
| Amiata | 10 (62.5) | 12 (75.0) | 6 (75.0) | 28 (70.0) | 11 (68.7) | 11 (68.7) | 8 (100.0) | 30 (75.0) | 21 (65.6) | 23 (71.9) | 14 (87.5) | 58 (72.5) |
| Viterbese | 15 (88.2) | 11 (68.8) | 6 (66.7) | 32 (76.2) | 16 (94.1) | 15 (93.8) | 9 (100.0) | 40 (95.2) | 31 (91.2) | 26 (81.3) | 15 (83.3) | 72 (85.7) |
| Taranto | 11 (84.6) | 9 (69.2) | 4 (66.7) | 24 (75.0) | 11 (84.6) | 10 (76.9) | 5 (83.3) | 26 (81.3) | 22 (84.6) | 19 (73.1) | 9 (75.0) | 50 (78.1) |
| Gela | 16 (69.6) | 20 (86.9) | 12 (100.0) | 48 (82.8) | 23 (100.0) | 12 (52.2) | 8 (66.7) | 43 (74.1) | 39 (84.8) | 32 (69.6) | 20 (83.3) | 91 (78.4) |
| Total | 52 (75.4) | 52 (76.5) | 28 (80.0) | 132 (75.9) | 61 (88.4) | 48 (70.6) | 30 (85.7) | 139 (80.8) | 113 (81.9) | 100 (73.5) | 58 (82.6) | 271 (78.8) |
| Statistics | Amiata | Viterbese | Taranto | Gela | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M + F | M | F | M + F | M | F | M + F | M | F | M + F | M | F | M + F | |
| n | 28 | 30 | 58 | 32 | 40 | 72 | 24 | 26 | 50 | 48 | 42 | 91 | 132 | 139 | 271 |
| AM | 10.23 | 6.24 | 8.16 | 12.89 | 13.37 | 13.15 | 24.24 | 30.23 | 27.36 | 29.89 | 24.76 | 27.47 | 20.57 | 18.51 | 19.51 |
| GM | 4.70 | 3.66 | 4.13 | 8.90 | 6.91 | 7.73 | 12.87 | 12.68 | 12.77 | 16.29 | 9.64 | 12.68 | 10.35 | 7.47 | 8.76 |
| SD | 19.54 | 7.96 | 14.73 | 12.18 | 28.55 | 22.64 | 34.34 | 56.57 | 46.86 | 30.07 | 32.96 | 31.26 | 26.77 | 35.03 | 31.24 |
| 5p | 1.30 | 1.32 | 1.26 | 3.67 | 1.99 | 2.23 | 3.54 | 2.62 | 2.72 | 2.36 | 1.19 | 1.46 | 1.73 | 1.42 | 1.50 |
| 25p | 2.65 | 1.75 | 1.92 | 5.58 | 4.20 | 5.15 | 7.92 | 5.93 | 6.64 | 7.23 | 3.58 | 4.51 | 4.42 | 3.27 | 3.71 |
| 50p | 3.22 | 2.42 | 3.02 | 7.71 | 5.80 | 7.05 | 10.69 | 9.91 | 10.25 | 10.96 | 6.52 | 9.95 | 8.81 | 5.99 | 7.78 |
| 75p | 8.78 | 8.27 | 8.27 | 14.50 | 11.07 | 11.83 | 31.33 | 23.15 | 28.68 | 55.07 | 37.12 | 45.65 | 28.21 | 12.47 | 16.37 |
| 95p | 37.37 | 26.92 | 32.49 | 42.58 | 46.04 | 42.58 | 72.79 | 155.91 | 155.91 | 88.68 | 90.08 | 89.07 | 73.37 | 90.08 | 86.28 |
| Factors Selected (p < 0.2) | Class | GM Exp. | 90% CI | GMR | 90% CI |
|---|---|---|---|---|---|
| Area | Amiata | 3.86 | 3.05–4.89 | 1 (reference) | |
| Viterbese | 8.60 | 6.93–10.68 | 2.23 | 1.62–3.06 | |
| Taranto | 11.75 | 8.82–15.66 | 3.05 | 2.08–4.46 | |
| Gela | 13.42 | 11.02–16.34 | 3.48 | 2.54–4.76 | |
| GSTT | - | 12.02 | 9.56–15.13 | 1 (reference) | |
| + | 8.12 | 7.21–9.15 | 0.68 | 0.52–0.88 | |
| Occupational exposure in chemical industrials | No | 8.17 | 7.31–9.12 | 1 (reference) | |
| Yes | 21.87 | 14.92–32.05 | 2.68 | 1.79–4.00 | |
| Seafood | No | 6.62 | 5.24–8.37 | 1 (reference) | |
| Yes | 9.57 | 8.49–10.78 | 1.44 | 1.11–1.88 | |
| Seafood consumption 3 days before urine collection | No | 7.75 | 6.86–8.75 | 1 (reference) | |
| Yes | 13.66 | 10.95–17.03 | 1.76 | 1.37–2.27 | |
| Whole milk | No | 8.25 | 7.30–9.31 | 1 (reference) | |
| Yes | 11.06 | 8.88–13.77 | 1.34 | 1.04–1.73 | |
| Wine | No | 8.69 | 7.80–9.67 | 1 (reference) | |
| Yes | 13.88 | 8.17–23.58 | 1.60 | 0.93–2.75 | |
| Meat | No | 7.96 | 6.83–9.27 | 1 (reference) | |
| Yes | 9.85 | 8.45–11.47 | 1.24 | 0.99–1.54 | |
| Whole milk of own/local production | No | 8.64 | 7.76–9.61 | 1 (reference) | |
| Yes | 18.25 | 9.90–33.64 | 2.11 | 1.13–3.94 | |
| Fruit/Vegetables of own/local production | No | 8.06 | 7.05–9.23 | 1 (reference) | |
| Yes | 11.03 | 8.80–13.82 | 1.37 | 1.03–1.82 |
| Factors Selected (p < 0.2) | Class | GM Exp. | 90% CI | GMR | 90% CI |
|---|---|---|---|---|---|
| GSTT | - | 5.57 | 3.72–8.32 | 1 (reference) | |
| + | 3.21 | 2.54–4.06 | 0.58 | 0.36–0.91 | |
| Smoker | No | 3.10 | 2.44–3.94 | 1 (reference) | |
| Yes | 4.97 | 3.43–7.22 | 1.61 | 1.04–2.48 | |
| Seafood consumption 3 days before urine collection | No | 3.02 | 2.40–3.81 | 1 (reference) | |
| Yes | 6.56 | 4.37–9.84 | 2.17 | 1.37–3.43 | |
| Meat | No | 2.85 | 2.07–3.91 | 1 (reference) | |
| Yes | 4.62 | 3.55–6.00 | 1.62 | 1.07–2.45 | |
| Whole milk of own/local production | No | 3.51 | 2.85–4.34 | 1 (reference) | |
| Yes | 9.17 | 5.13–16.38 | 2.61 | 1.42–4.79 |
| Factors Selected (p < 0.2) | Class | GM Exp. | 90% CI | GMR | 90% CI |
|---|---|---|---|---|---|
| Tap water | No | 6.85 | 5.41–8.68 | 1 (reference) | |
| Yes | 9.23 | 7.53–11.31 | 1.35 | 1.02–1.77 | |
| Smoker | No | 7.50 | 6.15–9.16 | 1 (reference) | |
| Yes | 10.55 | 8.24–13.51 | 1.41 | 1.06–1.86 | |
| Exposure to inorganic solvents and acids | No | 6.90 | 5.93–8.02 | 1 (reference) | |
| Yes | 19.42 | 9.89–38.14 | 2.81 | 1.40–5.67 | |
| Whole milk | No | 7.77 | 6.42–9.39 | 1 (reference) | |
| Yes | 10.75 | 8.23–14.04 | 1.38 | 1.04–1.84 | |
| Seafood consumption 3 days before urine collection | No | 6.46 | 5.29–7.88 | 1 (reference) | |
| Yes | 19.75 | 15.18–25.70 | 3.06 | 2.25–4.16 |
| Factors Selected (p < 0.2) | Class | GM Exp. | 90% CI | GMR | 90% CI |
|---|---|---|---|---|---|
| AS3MT | No | 5.64 | 3.59–8.87 | 1 (reference) | |
| Yes | 8.19 | 4.84–13.87 | 1.45 | 0.92–2.30 | |
| Tap water | No | 3.48 | 1.43–8.47 | 1 (reference) | |
| Yes | 8.29 | 5.66–12.13 | 2.38 | 0.99–5.73 | |
| Exposure to inorganic solvents and acids | No | 5.17 | 3.31–8.07 | 1 (reference) | |
| Yes | 14.81 | 8.28–26.50 | 2.86 | 1.69–4.84 | |
| Seafood | No | 3.56 | 1.78–7.12 | 1 (reference) | |
| Yes | 7.34 | 4.83–11.15 | 2.06 | 1.15–3.71 | |
| Meat | No | 4.78 | 3.20–7.14 | 1 (reference) | |
| Yes | 8.31 | 4.73–14.61 | 1.74 | 1.07–2.81 | |
| Bread, pasta, cereals | No | 1.85 | 0.64–5.39 | 1 (reference) | |
| Yes | 6.61 | 4.34–10.08 | 3.57 | 1.40–9.10 | |
| Whole milk | No | 4.81 | 3.11–7.45 | 1 (reference) | |
| Yes | 14.75 | 7.99–27.23 | 3.06 | 1.78–5.26 | |
| Coffee | No | 3.43 | 1.97–5.97 | 1 (reference) | |
| Yes | 7.64 | 4.81–12.14 | 2.23 | 1.29–3.84 | |
| Fruit/Vegetables of own/local production | No | 4.95 | 2.87–8.53 | 1 (reference) | |
| Yes | 11.17 | 7.92–15.77 | 2.26 | 1.31–3.88 |
| Factors Selected (p < 0.2) | Class | GM Exp. | 90% CI | GMR | 90% CI |
|---|---|---|---|---|---|
| GSTT | - | 22.43 | 14.12–35.62 | 1 (reference) | |
| + | 9.68 | 7.65–12.25 | 0.43 | 0.26–0.72 | |
| Occupational exposure in chemical industrials | No | 10.48 | 8.34–13.17 | 1 (reference) | |
| Yes | 36.67 | 23.15–58.08 | 3.50 | 2.09–5.85 | |
| Seafood | No | 6.89 | 4.55–10.44 | 1 (reference) | |
| Yes | 13.37 | 10.48–17.04 | 1.94 | 1.21–3.12 | |
| Coffee | No | 19.86 | 13.45–29.31 | 1 (reference) | |
| Yes | 9.81 | 7.66–12.57 | 0.49 | 0.31–0.78 |
| Country | Acronym of the Study | Year of Recruitment | Age Class | n | GM | 50p | 95p | Reference |
|---|---|---|---|---|---|---|---|---|
| Italy | SEpiAs | 2010 | 20–44 | 271 | 8.76 | 7.78 | 86.28 | [49] |
| Germany | GerES-III | 1998 | 18–69 25–69 | 4741 4052 | 3.92 3.87 | 4.1 4.0 | 18.9 19.3 | [90,91] |
| France | ENNS | 2006–2007 | 18–39 18–74 | 444 1500 | 4.07 3.75 | 4.49 4.03 | 10.72 10.68 | [92] |
| France | ENNS | 2006–2007 | 18–74 | 3015 | 3.75 | nr | nr | [83] |
| USA | NHANES | 2009–2010 2011–2012 | >20 years | 2020 1724 | 6.7 5.6 | 5.95 5.15 | 23.2 17.6 | [57] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minichilli, F.; Bianchi, F.; Ronchi, A.M.; Gorini, F.; Bustaffa, E. Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy. Int. J. Environ. Res. Public Health 2018, 15, 299. https://doi.org/10.3390/ijerph15020299
Minichilli F, Bianchi F, Ronchi AM, Gorini F, Bustaffa E. Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy. International Journal of Environmental Research and Public Health. 2018; 15(2):299. https://doi.org/10.3390/ijerph15020299
Chicago/Turabian StyleMinichilli, Fabrizio, Fabrizio Bianchi, Anna Maria Ronchi, Francesca Gorini, and Elisa Bustaffa. 2018. "Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy" International Journal of Environmental Research and Public Health 15, no. 2: 299. https://doi.org/10.3390/ijerph15020299
APA StyleMinichilli, F., Bianchi, F., Ronchi, A. M., Gorini, F., & Bustaffa, E. (2018). Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy. International Journal of Environmental Research and Public Health, 15(2), 299. https://doi.org/10.3390/ijerph15020299







