Focus on Chronic Exposure for Deriving Drinking Water Guidance Underestimates Potential Risk to Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hazard Assessment and Dose–Response
Reference Dose Derivation and Selection
2.2. Exposure Assessment—Drinking Water Intake Rates and the RSC
2.3. Risk Characterization
- nHBGduration = the noncancer health-based guidance value, for a given duration (see Table 1), expressed in units of micrograms of chemical per liter of water (µg/L).
- RfDduration = the reference dose, for a given duration (see Table 1), expressed in units of milligrams of the chemical per kilogram of body weight per day (mg/kg per day).
- RSC = the relative source contribution factor, as described above.
- IRduration = the intake rate of water ingested for a given duration, as described above.
Final Selection of Health-Based Guidance (HBG)
3. Results and Discussion
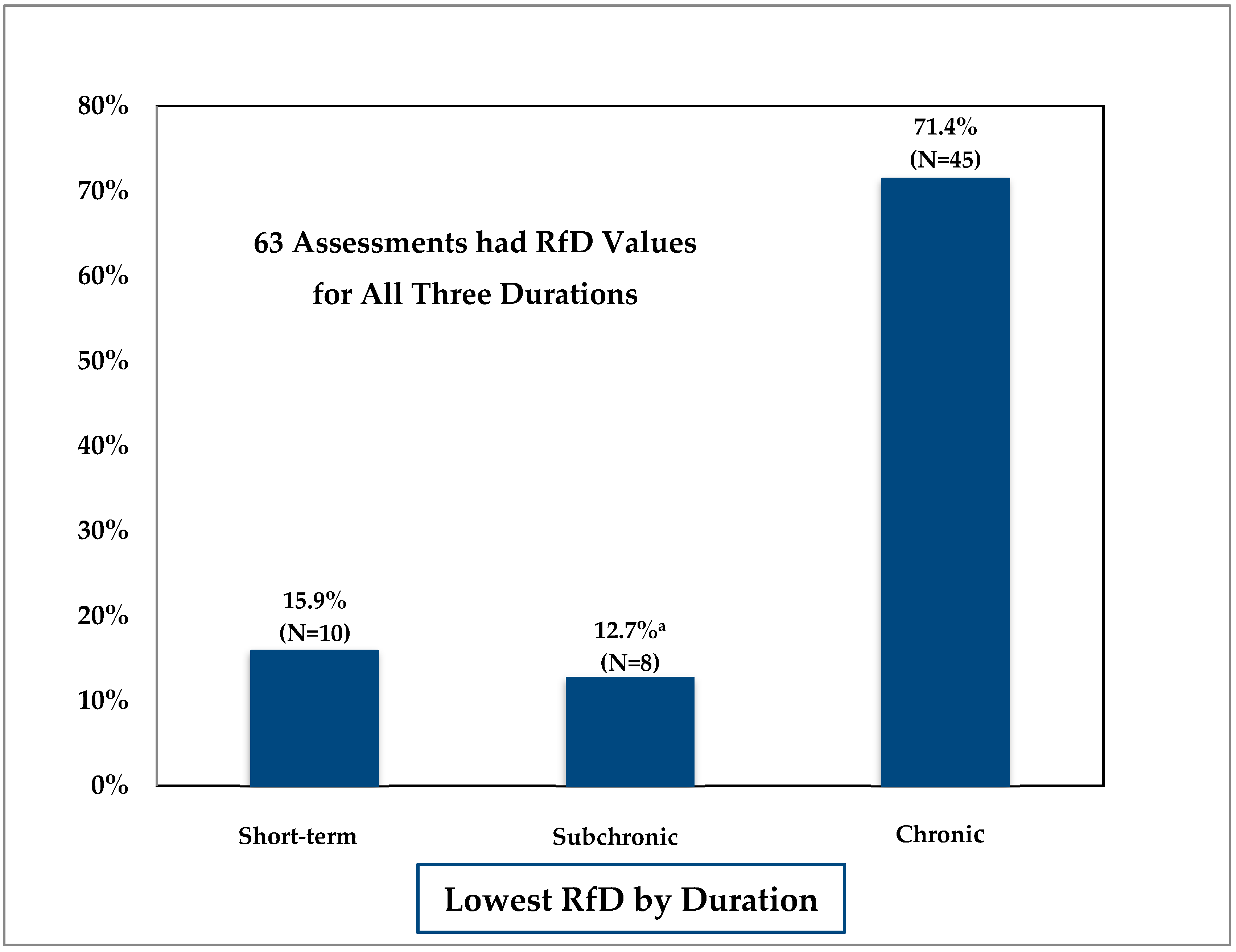
3.1. Comparison of Duration-Specific Reference Doses
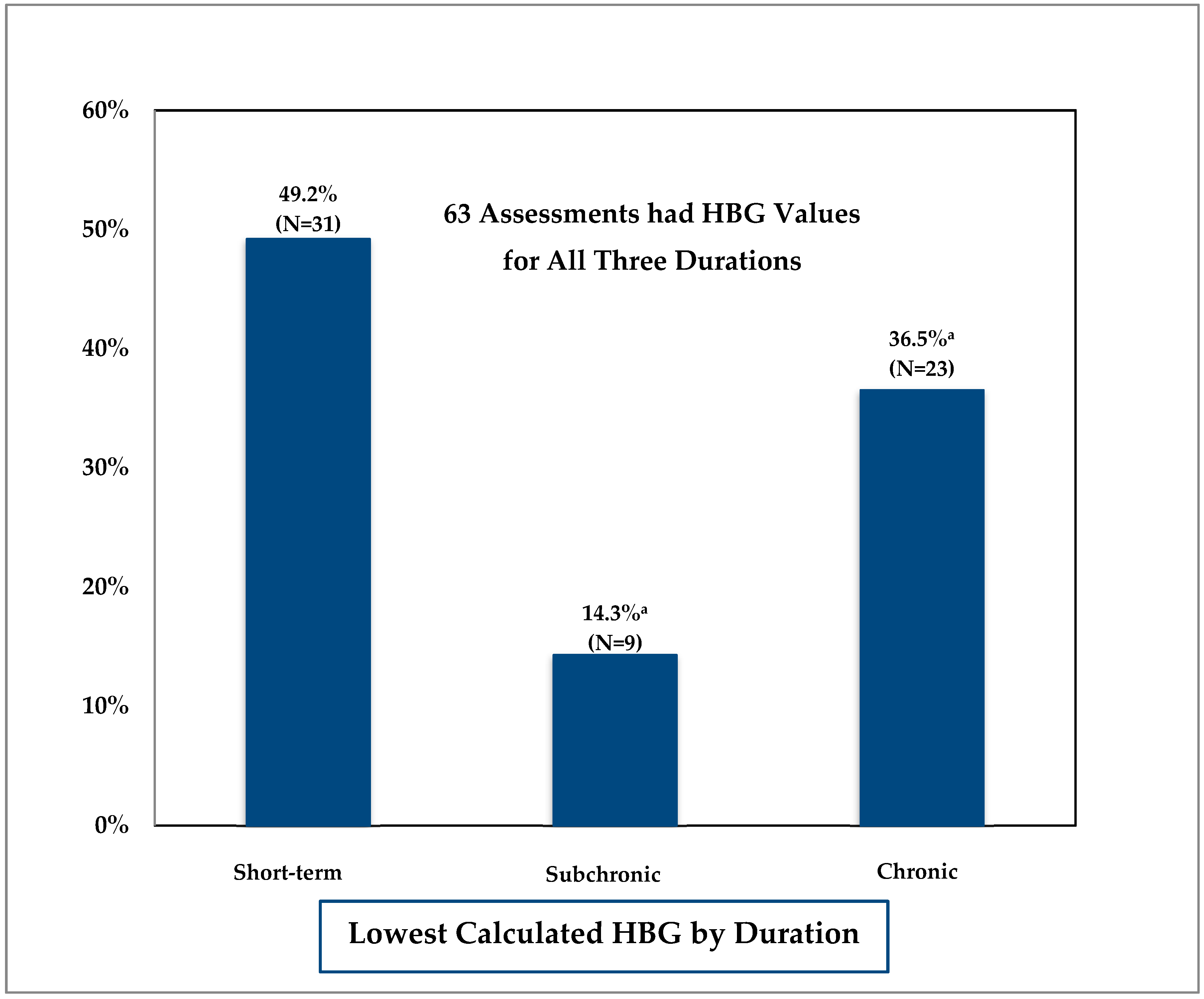
3.2. Comparison of Duration-Specific Health-Based Guidance (HBG) Values
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- National Academy of Sciences. Pesticides in the Diets of Infants and Children; National Academies Press: Washington, DC, USA, 1993; Available online: https://www.nap.edu/catalog/2126/pesticides-in-the-diets-of-infants-and-children (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). A Review of the Reference Dose and Reference Concentration Processes; EPA: Washington, DC, USA, 2002. Available online: https://www.epa.gov/osa/review-reference-dose-and-reference-concentration-processes (accessed on 13 March 2018).
- Minnesota Department of Health (MDH). Statement of Need and Reasonableness (SONAR), July 11, 2008. Support Document Relating to Health Risk Limits for Groundwater Rules; MDH: St Paul, MN, USA, 2008; Available online: http://www.health.state.mn.us/divs/eh/risk/rules/water/hrlsonar08.pdf (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants; EPA: Washington, DC, USA, 2005. Available online: https://www.epa.gov/sites/production/files/2013-09/documents/agegroups.pdf (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). A Framework for Assessing Health Risks of Environmental Exposures to Children; EPA: Washington, DC, USA, 2006. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=158363 (accessed on 13 March 2018).
- Minnesota Statutes. 144.0751 Health Standards. Available online: https://www.revisor.mn.gov/statutes?id=144.0751&year=2001&keyword_type=all&keyword=144.0751 (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Office of Water. 2012 Edition of the Drinking Water Standards and Health Advisories; EPA: Washington, DC, USA, 2012. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/dwstandards2012.pdf (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health; EPA-822-B-00-004; EPA: Washington, DC, USA, 2000. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/20003D2R.PDF?Dockey=20003D2R.PDF (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Human Health Risk Assessment; EPA: Washington, DC, USA. Available online: https://www.epa.gov/risk/human-health-risk-assessment (accessed on 13 March 2018).
- Minnesota Department of Health (MDH). MDH Health Risk Assessment Methods to Incorporate Human Equivalent Dose Calculations into Derivation of Oral Reference Doses; MDH: St Paul, MN, USA, 2011; (revised 2017); Available online: http://www.health.state.mn.us/divs/eh/risk/guidance/hedrefguide.pdf (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Toxicological Review of Benzo[a]pyrene; EPA: Washington, DC, USA, 2017. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0136tr.pdf (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Estimated Per Capita Water Ingestion and Body Weight in the United States—An Update. Based on Data Collected by the United States Department of Agriculture’s 1994–1996 and 1998 Continuing Survey of Food Intakes by Individuals; EPA: Washington, DC, USA, 2004.
- U.S. Environmental Protection Agency (EPA). An SAB Report on EPA’s Per Capita Water Ingestion in the United States; EPA-SAB-EC-00-003; EPA: Washington, DC, USA, 1999.
- U.S. Environmental Protection Agency (EPA). Exposure Factors Handbook. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 13 March 2018).
- Minnesota Statutes. 103H.201 Health Risk Limits; 1989. Available online: https://www.revisor.mn.gov/statutes?id=103H&year=1989#search="103H.201" (accessed on 13 March 2018).
- Batke, M.; Escher, S.; Hoffmann-Doerr, S.; Melber, C.; Messinger, H.; Mangelsdorf, I. Evaluation of time extrapolation factors based on the database RepDose. Toxicol. Lett. 2011, 205, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zarn, H.; Engeli, B.E.; Schlatter, J.R. Study parameters influencing NOAEL and LOAEL in toxicity feeding studies for pesticides: Exposure duration versus dose decrement, dose spacing, group size and chemical class. Regul. Toxicol. Pharmacol. 2011, 61, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Malkiewicz, K.; Hansson, S.O.; Ruden, C. Assessment factors for extrapolation from short-time to chronic exposure—Are the REACH guidelines adequate? Toxicol. Lett. 2009, 190, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Groenveld, C.; Hakkert, B.C.; Bos, P.M.J.; de Herr, C. Extrapolation exposure duration in Oral Toxicity: A quantitative analysis of historical Toxicity Data. Hum. Ecol. Risk Assess. 2004, 10, 709–716. [Google Scholar] [CrossRef]
- Kramer, H.; van den Ham, W.A.; Slob, W.; Pieters, M.N. Conversion Factors estimating Indicative Chronic No-Observed-Adverse-Effect levels from short-term Toxicity Data. Regul. Toxicol. Pharmacol. 1996, 23, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Bokkers, B.; Slob, W. A comparison of ratio distributions based on the NOAEL and the Benchmark Approach for Subchronic-to-Chronic Extrapolation. Toxicol. Sci. 2005, 85, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Kramer, H.J.; Slob, W. Evalution of the uncertainty factor for Subchronic-to-Chronic Extrapolation: Statistical analysis of Toxicity Data. Regul. Toxicol. Pharmacol. 1998, 27, 108–111. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). Memorandum: New Policy on Evaluating Health Risks to Children; EPA: Washington, DC, USA, 1995. Available online: https://www.epa.gov/sites/production/files/2014-05/documents/1995_childrens_health_policy_statement.pdf (accessed on 13 March 2018).
- U.S. Environmental Protection Agency (EPA). Memorandum: Reaffirmation of the U.S. Environmental Protection Agency’s 1995 Policy on Evaluating Health Risks to Children; EPA: Washington, DC, USA, 2013. Available online: https://www.epa.gov/sites/production/files/2014-05/documents/reaffirmation_memorandum.pdf (accessed on 13 March 2018).
- Cohen Hubal, E.; de Wet, T.; Toit, L.D.; Firestone, M.P.; Ruchirawat, M.; van Englen, J.; Vickers, C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul. Toxicol. Pharmacol. 2014, 69, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Department of Health (MDH). Report on Pesticide Rapid Assessments; MDH: St Paul, MN, USA, 2014; Available online: http://www.health.state.mn.us/divs/eh/risk/guidance/dwec/rapidpest.html (accessed on 13 March 2018).
- Minnesota Department of Health (MDH). Pharmaceutical Water Screening Values Report; MDH: St Paul, MN, USA, 2015; Available online: http://www.health.state.mn.us/divs/eh/risk/guidance/dwec/pharmproj.html (accessed on 13 March 2018).
| Duration | Definition |
|---|---|
| Short-term | Repeated exposure for more than 24 h, up to 30 days. |
| Subchronic | Repeated exposure for more than 30 days, up to approximately 10 percent of a lifetime (approximately 90 days in typical laboratory rodent studies) |
| Chronic | Repeated exposure for more than approximately 10 percent of a lifespan (more than 90 days, to 2 years in typical laboratory rodent studies) |
| Age Group | Mean (L/kg-Day) | Ratio to All Ages | 95th Percentile (L/kg-Day) | Ratio to All Ages |
|---|---|---|---|---|
| Birth to <1 month | 0.137 | 8.6 | 0.238 | 5.4 |
| 1 to <3 months | 0.119 | 7.4 | 0.285 | 6.5 |
| 3 to <6 months | 0.080 | 5.0 | 0.173 | 3.9 |
| 6 to <12 months | 0.053 | 3.3 | 0.129 | 2.9 |
| 1 to <2 years | 0.027 | 1.7 | 0.075 | 1.7 |
| 2 to <3 years | 0.026 | 1.6 | 0.062 | 1.4 |
| 3 to <6 years | 0.021 | 1.3 | 0.052 | 1.2 |
| 6 to <11 years | 0.017 | 1.1 | 0.047 | 1.1 |
| 11 to <16 years | 0.012 | 0.8 | 0.035 | 0.8 |
| 16 to <18 years | 0.010 | 0.6 | 0.030 | 0.7 |
| 18 to <21 years | 0.011 | 0.7 | 0.036 | 0.8 |
| ≥21 years | 0.016 | 1.0 | 0.042 | 1.0 |
| All ages (lifetime) | 0.016 | 0.044 | ||
| Pregnant women | 0.014 | 0.9 | 0.043 | 1.0 |
| Lactating women | 0.026 | 1.6 | 0.055 | 1.3 |
| Study | Comparison Parameter a | Number of Chemicals | Geometric Mean ± GSD | 95th Percentile |
|---|---|---|---|---|
| Short-term to Chronic | ||||
| Current Analysis | RfD | 63 | 3.5 ± 2.8 | 24.1 |
| RfD b | 18 | 2.9 ± 2.4 | 17.1 | |
| Batke et al. [16] | NOAEL | 14 | 3.4 ± 3.7 | 29.2 |
| Zarn et al. [17] | NOAEL (rat) | 107 | 4.3 ± 4.7 | 53.2 |
| NOAEL (mouse) | 56 | 3.4 ± 3.6 | 23.7 | |
| Malkiewicz et al. [18] | NOAEL | 26 | 3.1 ± 2.1 | |
| Groeneveld et al. [19] | NOAEL | 35 | 4.9 ± 3.5 | 38.6 |
| Kramer et al. [20] | NOAEL | 71 | 4.1 ± 4.4 | 46 |
| Subchronic to Chronic | ||||
| Current Analysis | RfD | 63 | 1.9 ± 2.0 | 5.4 |
| RfD b | 18 | 1.6 ± 2.0 | 4.6 | |
| Batke et al. [16] | NOAEL | 58 | 1.4 ± 2.1 | 4.7 |
| Zarn et al. [17] | NOAEL (rat) NOAEL (mouse) | 222 99 | 2.5 ± 3.4 2.2 ± 3.9 | 17.4 21.4 |
| Malkiewicz et al. [18] | NOAEL | 32 | 2.3 ± 2.0 | |
| Bokkers and Slob [21] | NOAEL benchmark dose | 68 189 | 1.5 ± 5.3 1.7 ± 2.9 | 22.7 9.9 |
| Groeneveld et al. [19] | NOAEL | 70 | 2.3 ± 3.6 | 18.4 |
| Pieters et al. [22] | NOAEL | 149 | 1.7 ± 5.6 | 29 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goeden, H. Focus on Chronic Exposure for Deriving Drinking Water Guidance Underestimates Potential Risk to Infants. Int. J. Environ. Res. Public Health 2018, 15, 512. https://doi.org/10.3390/ijerph15030512
Goeden H. Focus on Chronic Exposure for Deriving Drinking Water Guidance Underestimates Potential Risk to Infants. International Journal of Environmental Research and Public Health. 2018; 15(3):512. https://doi.org/10.3390/ijerph15030512
Chicago/Turabian StyleGoeden, Helen. 2018. "Focus on Chronic Exposure for Deriving Drinking Water Guidance Underestimates Potential Risk to Infants" International Journal of Environmental Research and Public Health 15, no. 3: 512. https://doi.org/10.3390/ijerph15030512




