Association between Short-Term Exposure to Air Pollution and Dyslipidemias among Type 2 Diabetic Patients in Northwest China: A Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Air Pollution and Weather Data
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| CVD | Cardiovascular diseases |
| TC | Total cholesterol |
| TG | Triglycerides |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| FPG | Fasting plasma glucose |
| BMI | Body mass index |
| GM | Geometric mean |
References
- Sun, Q.; Hong, X.; Wold, L.E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 2010, 121, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Zhang, J.; Zhong, N. Outdoor air pollution and respiratory health in asia. Respirology 2011, 16, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.L.; Mittleman, M.A. Ambient air pollution and stroke. Stroke 2014, 45, 3734–3741. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.B.; Ravnskjaer, L.; Loft, S.; Andersen, K.K.; Brauner, E.V.; Baastrup, R.; Yao, C.; Ketzel, M.; Becker, T.; Brandt, J.; et al. Long-term exposure to fine particulate matter and incidence of diabetes in the danish nurse cohort. Environ. Int. 2016, 91, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, C.; Zhao, Y.; Ma, Z.; Bi, J.; Liu, Y.; Meng, X.; Wang, Y.; Cai, J.; Kan, H.; et al. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in china. Environ. Int. 2016, 92–93, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Cakmak, S.; Turner, M.C.; Brook, J.R.; Crouse, D.L.; Peters, P.A.; van Donkelaar, A.; Villeneuve, P.J.; Brion, O.; Jerrett, M.; et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013, 36, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. Cardiovascular damage by airborne particles: Are diabetics more susceptible? Epidemiology 2002, 13, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; Gallina, P.; Pais, M.; Cazzolato, G.; Alessandrini, P.; Bittolo-Bon, G. Hypertriglyceridemia is associated with increased insulin resistance in subjects with normal glucose tolerance: Evaluation in a large cohort of subjects assessed with the 1999 world health organization criteria for the classification of diabetes. Metabolism 2003, 52, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.; Hillege, H.L.; Stolk, R.P.; Navis, G.; Bakker, S.J.; Dullaart, R.P. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: The prevend study. J. Clin. Endocrinol. Metab. 2013, 98, E1352–E1359. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diabetes 1996 Vital Statistics; American Diabetes Assoiciation: Alexandria, VA, USA, 1996. [Google Scholar]
- Yitshak Sade, M.; Kloog, I.; Liberty, I.F.; Schwartz, J.; Novack, V. The association between air pollution exposure and glucose and lipids levels. J. Clin. Endocrinol. Metab. 2016, 101, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Salam, M.T.; Toledo-Corral, C.; Watanabe, R.M.; Xiang, A.H.; Buchanan, T.A.; Habre, R.; Bastain, T.M.; Lurmann, F.; Wilson, J.P.; et al. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 2016, 39, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Shanley, R.P.; Hayes, R.B.; Cromar, K.R.; Ito, K.; Gordon, T.; Ahn, J. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 2016, 27, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, E.R.; Stafoggia, M.; Faustini, A.; Berti, G.; Canova, C.; De Togni, A.; Di Biagio, K.; Gherardi, B.; Giannini, S.; Lauriola, P.; et al. Association between short-term exposure to PM2.5 and PM10 and mortality in susceptible subgroups: A multisite case-crossover analysis of individual effect modifiers. Am. J. Epidemiol. 2016. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; Zhou, Y.; Chen, L.; Chen, S.; Yao, T.; Qin, T.; Wu, S. The effect of air pollution on human physiological function in china: A longitudinal study. The Lancet 2015, 386, S31. [Google Scholar] [CrossRef]
- Coogan, P.F.; White, L.F.; Yu, J.; Burnett, R.T.; Seto, E.; Brook, R.D.; Palmer, J.R.; Rosenberg, L.; Jerrett, M. Pm2.5 and diabetes and hypertension incidence in the black women’s health study. Epidemiology 2016, 27, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yang, A.; Pu, H.; Dai, M.; Cheng, N.; Ding, J.; Li, J.; Li, H.; Hu, X.; Ren, X.; et al. Cohort profile: The china metal-exposed workers cohort study (Jinchang cohort). Int. J. Epidemiol. 2017, 46, 1095–1096e. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Y.; Li, S.; Williams, G.; Kan, H.; Marks, G.B.; Morawska, L.; Abramson, M.J.; Chen, S.; Yao, T.; et al. Air pollution and fasting blood glucose: A longitudinal study in China. Sci. Total Environ. 2016, 541, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Khafaie, M.A.; Salvi, S.S.; Ojha, A.; Khafaie, B.; Gore, S.S.; Yajnik, C.S. Systemic inflammation (C-reactive protein) in type 2 diabetic patients is associated with ambient air pollution in Pune City, India. Diabetes Care 2013, 36, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Marcello, A.; Giovanni, P.; Carla, F. Mechanisms of diabetic dyslipidemia: Relevance for atherogenesis. Curr. Vasc. Pharmacol. 2012, 10, 684–686. [Google Scholar]
- Lin, D.; Qi, Y.; Huang, C.; Wu, M.; Wang, C.; Li, F.; Yang, C.; Yan, L.; Ren, M.; Sun, K. Associations of lipid parameters with insulin resistance and diabetes: A population-based study. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Eze, I.C.; Hemkens, L.G.; Bucher, H.C.; Hoffmann, B.; Schindler, C.; Kunzli, N.; Schikowski, T.; Probst-Hensch, N.M. Association between ambient air pollution and diabetes mellitus in europe and North America: Systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Li, K.; Zhou, Q. The association between air pollutants and morbidity for diabetes and liver diseases modified by sexes, ages, and seasons in Tianjin, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Jia, J.; Chen, B. The association of daily diabetes mortality and outdoor air pollution in Shanghai, China. J. Environ. Health 2004, 67, 21–26. [Google Scholar] [PubMed]
- Brook, R.D.; Sun, Z.; Brook, J.R.; Zhao, X.; Ruan, Y.; Yan, J.; Mukherjee, B.; Rao, X.; Duan, F.; Sun, L.; et al. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: The air pollution and cardiometabolic disease study. Hypertension 2016, 67, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Hansell, A.L.; Blangiardo, M.; Burton, P.R.; de Hoogh, K.; Doiron, D.; Fortier, I.; Gulliver, J.; Hveem, K.; Mbatchou, S.; et al. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the hunt and lifelines cohorts. Eur. Heart J. 2017, 38, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Poursafa, P.; Mansourian, M.; Motlagh, M.E.; Ardalan, G.; Kelishadi, R. Is air quality index associated with cardiometabolic risk factors in adolescents? The CASPIAN-III study. Environ. Res. 2014, 134, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bind, M.A.; Peters, A.; Koutrakis, P.; Coull, B.; Vokonas, P.; Schwartz, J. Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly american men: The normative aging study. Environ. Health Perspect. 2016, 124, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Lovati, M.R.; Manzoni, C.; Daldossi, M.; Spolti, S.; Sirtori, C.R. Effects of sub-chronic exposure to SO2 on lipid and carbohydrate metabolism in rats. Arch. Toxicol. 1996, 70, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Raaschou-Nielsen, O.; Ketzel, M.; Jensen, S.S.; Hvidberg, M.; Loft, S.; Tjønneland, A.; Overvad, K.; Sørensen, M. Diabetes incidence and long-term exposure to air pollution: A cohort study. Diabetes Care 2012, 35, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Cakmak, S.; Vidal, C.B.; Rubio, M.A. Air pollution and hospitalization for acute complications of diabetes in chile. Environ. Int. 2012, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Hong, Y.C.; Kim, H. Effect of changes in season and temperature on mortality associated with air pollution in Seoul, Korea. J. Epidemiol. Community Health 2011, 65, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; London, S.J.; Chen, G.; Zhang, Y.; Song, G.; Zhao, N.; Jiang, L.; Chen, B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The public health and air pollution in asia (papa) study. Environ. Health Perspect. 2008, 116, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Poggio, R.; Elorriaga, N.; Gutierrez, L.; Irazola, V.; Rubinstein, A.; Danaei, G. Associations between dietary patterns and serum lipids, apo and C-reactive protein in an adult population: Evidence from a multi-city cohort in South America. Br. J. Nutr. 2017, 117, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Jayashankar, C.A.; Andrews, H.P.; Vijayasarathi; Pinnelli, V.B.; Shashidharan, B.; Nithin Kumar, H.N.; Vemulapalli, S. Serum uric acid and low-density lipoprotein cholesterol levels are independent predictors of coronary artery disease in Asian Indian patients with type 2 diabetes mellitus. J. Nat. Sci. Biol. Med. 2016, 7, 161–165. [Google Scholar] [CrossRef] [PubMed]
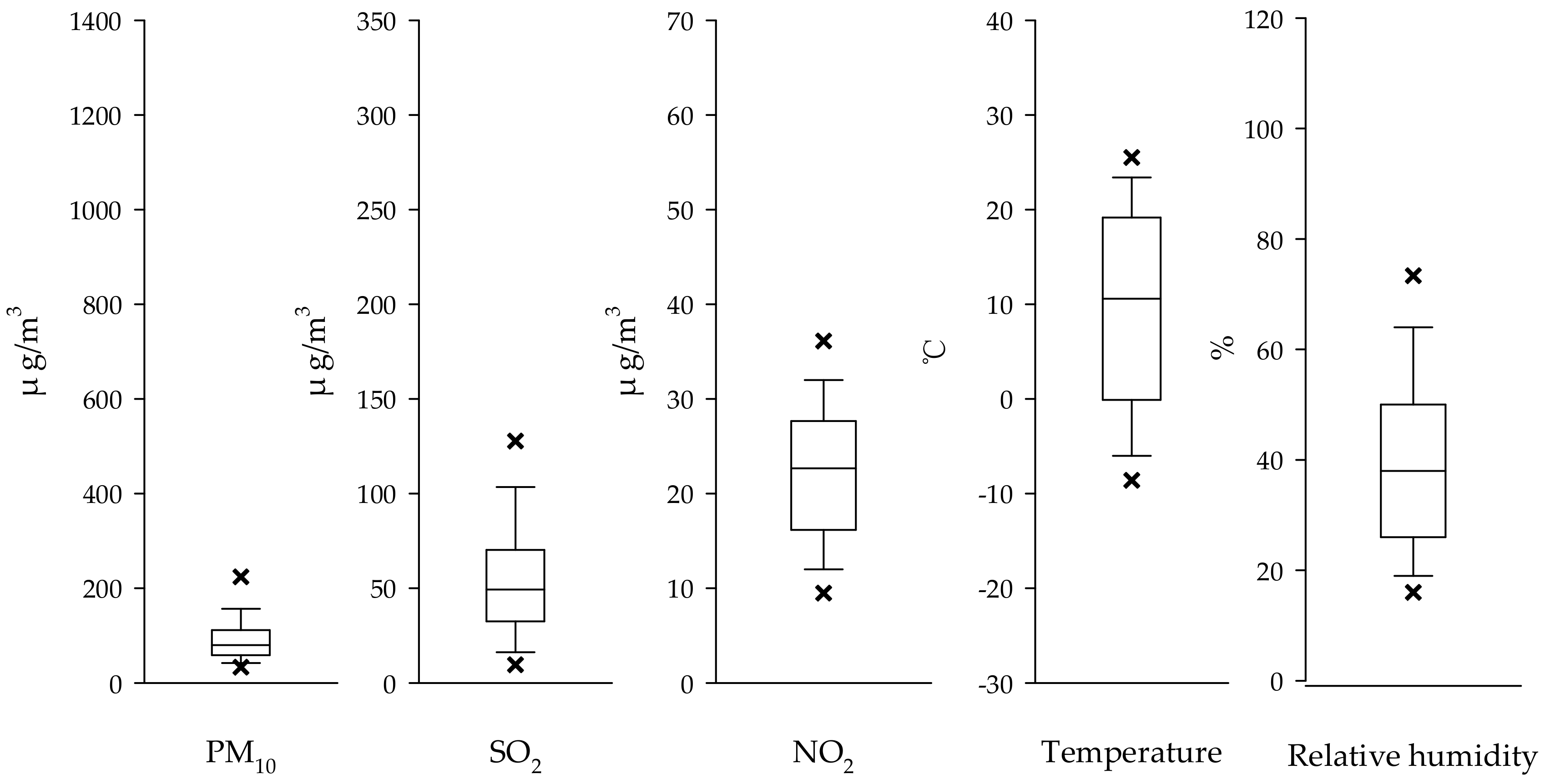

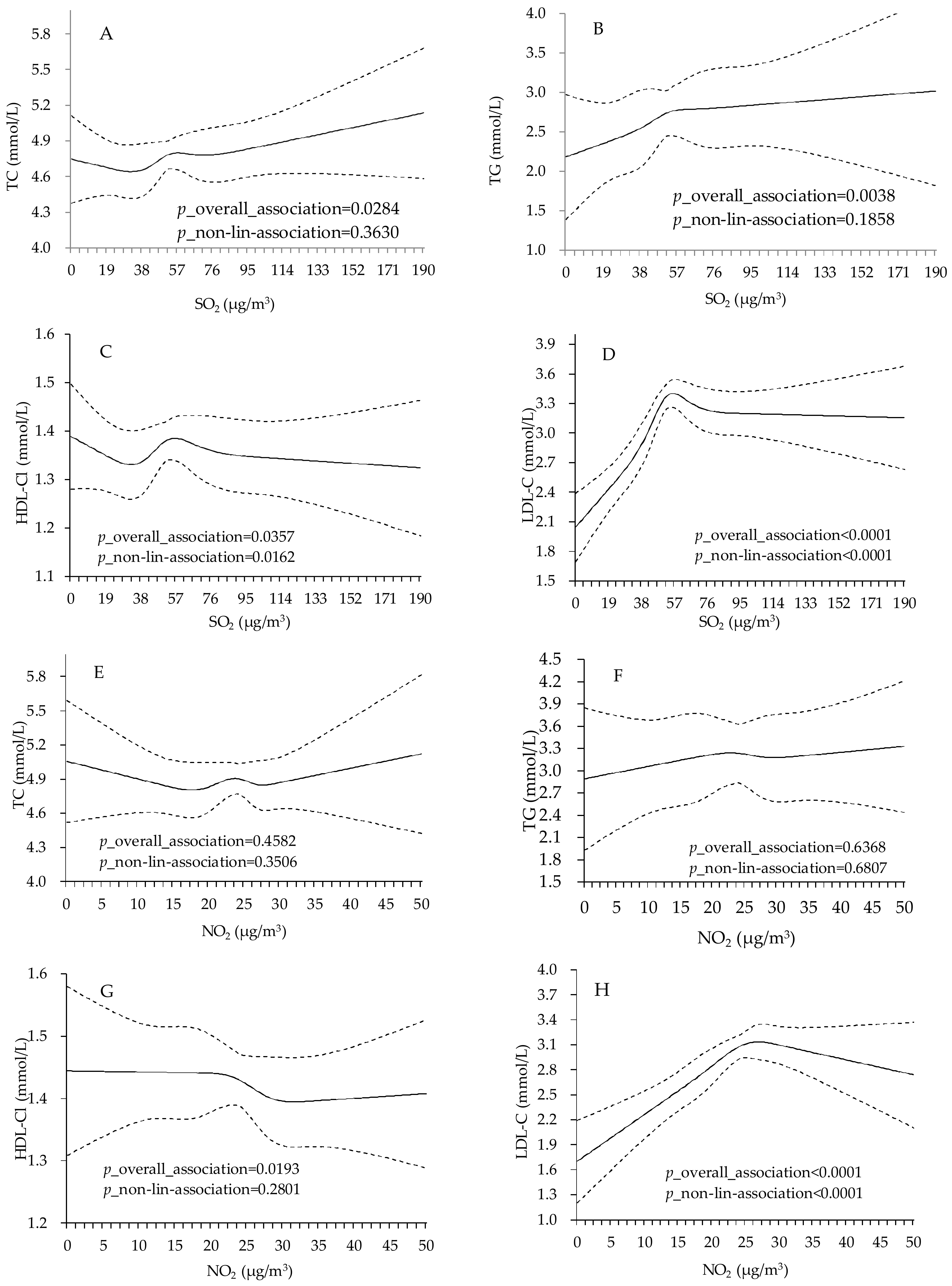
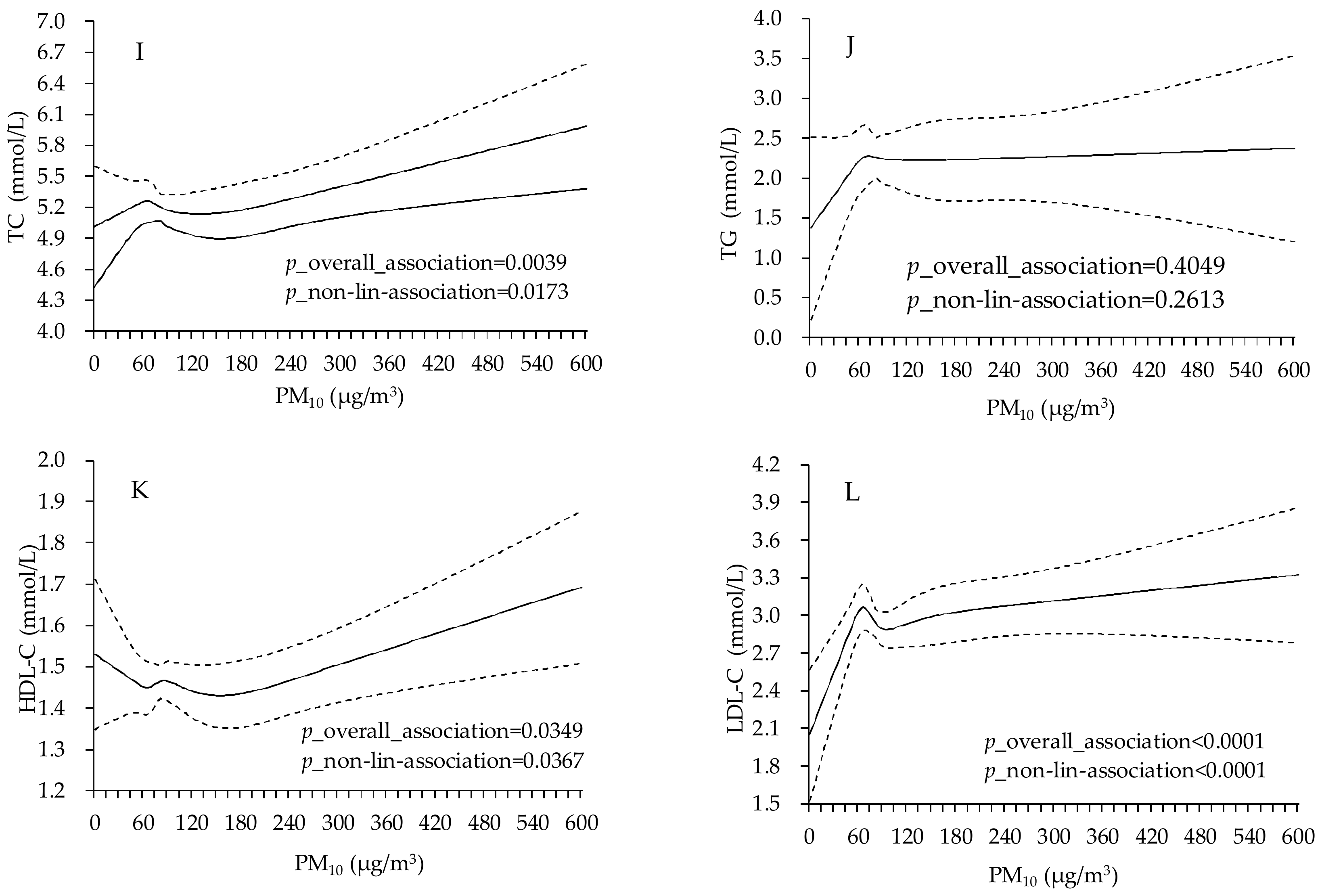
| Variable | Total | Male | Female |
|---|---|---|---|
| N (n, %) | 3912 (100) | 2751 (70.32) | 1161 (29.68) |
| Age (n, %) | |||
| <60 years | 1869 (47.78) | 1368 (49.73) | 501 (43.15) |
| ≥60 years | 2043 (52.22) | 1383 (50.27) | 660 (56.85) |
| Education (n, %) | |||
| No normal education | 1192 (30.47) | 782 (28.43) | 410 (35.31) |
| Primary education | 1225 (31.31) | 834 (30.32) | 391 (33.68) |
| Middle school | 866 (22.14) | 610 (22.17) | 256 (22.05) |
| High school | 380 (9.71) | 314 (11.41) | 66 (5.68) |
| College or higher | 249 (6.37) | 211 (7.67) | 38 (3.24) |
| Work type (n, %) | |||
| Management and services | 1166 (29.81) | 741 (26.94) | 425 (36.61) |
| workers | 2746 (70.19) | 2010 (73.06) | 736 (63.39) |
| Married (n, %) | 3387 (86.58) | 2484 (90.29) | 903 (77.78) |
| Smoking (n, %) | |||
| Never | 1807 (46.19) | 697 (25.34) | 1110 (95.61) |
| Current smoker | 1400 (35.79) | 1364 (49.58) | 36 (3.10) |
| Former smoker | 705 (18.02) | 690 (25.08) | 15 (1.29) |
| Drinking (n, %) | |||
| Never | 2800 (71.57) | 1662 (60.41) | 1138 (98.02) |
| Current drinker | 701 (17.92) | 682 (24.79) | 19 (1.64) |
| Former drinker | 411 (10.51) | 407 (14.79) | 4 (0.34) |
| Hypertension (n, %) | 1928 (49.28) | 1320 (47.98) | 608 (52.37) |
| BMI (kg/m2) (mean, SD) | 25.26 (3.14) | 25.14 (2.94) | 25.54 (3.53) |
| Total cholesterol (TC) (mmol/L) (median, 25th, 75th) | 4.80 (4.20, 5.50) | 4.70 (4.10, 5.40) | 5.00 (4.40, 5.80) |
| Triglycerides (TG) (mmol/L) (median, 25th, 75th) | 1.90 (1.40, 2.90) | 1.97 (1.40, 3.00) | 1.80 (1.30, 2.60) |
| High-density lipoprotein cholesterol (HDL-C) (mmol/L) (median, 25th, 75th) | 1.20 (1.02, 1.41) | 1.16 (0.99, 1.37) | 1.29 (1.11, 1.52) |
| Low-density lipoprotein cholesterol (LDL-C) (mmol/L) (median, 25th, 75th) | 3.03 (2.43, 3.61) | 3.04 (2.49, 3.59) | 3.01 (2.26, 3.65) |
| FPG (mmol/L) (median, 25th, 75th) | 8.00 (7.10, 9.80) | 8.00 (7.20, 9.90) | 8.00 (7.10, 9.70) |
| Air Pollutants | Models | TC | TG | HDL-C | LDL-C |
|---|---|---|---|---|---|
| % Change in GM (95% CI) | % Change in GM (95% CI) | % Change in GM (95% CI) | % Change in GM (95% CI) | ||
| SO2 | Model1 a | 1.21 (0.38, 2.04) * | 3.97 (1.58, 6.41) * | 0.13 (−0.70, 0.98) | 8.78 (7.26, 10.33) * |
| Model2 b | 1.31 (0.40, 2.12) * | 3.52 (1.07, 6.03) * | 0.59 (−0.31, 1.51) | 7.53 (5.98, 9.09) * | |
| PM10 | Model1 a | 0.49 (0.13, 0.85) * | 0.03 (−0.94, 1.01) | 0.31 (0.12, 0.51) * | 0.65 (0.05, 1.26) * |
| Model2 b | 0.45 (0.08, 0.82) * | −0.01 (−1.01, 1.00) | 0.29 (0.10, 0.49) * | 0.83 (0.21, 1.45) * | |
| NO2 | Model1 a | 1.34 (−0.86, 3.60) | 6.28 (0.08, 12.87) * | −2.91 (−5.46, −0.30) * | 43.63 (36.02, 51.67) * |
| Model2 b | 1.16 (−1.06, 3.43) | 5.58 (−0.62, 12.16) | −3.55 (−6.40, −0.61) * | 39.01 (31.43, 47.03) * |
| Groups | Air Pollutants | TC | TG | HDL-C | LDL-C |
|---|---|---|---|---|---|
| % Change in GM (95% CI) | % Change in GM (95% CI) | % Change in GM (95% CI) | % Change in GM (95% CI) | ||
| Gender | |||||
| Male | SO2 | 1.15 (0.19, 2.13) * | 4.39 (1.50, 7.36) * | −0.26 (−1.25, 0.74) | 7.49 (5.81, 9.20) * |
| PM10 | 0.59 (0.09, 1.09) * | −0.18 (−1.57, 1.23) | 0.28 (0.00, 0.57) * | 0.47 (−0.33, 1.27) | |
| NO2 | 1.90 (−0.86, 4.74) | 5.77 (−2.12, 14.30) | −2.72 (−5.81, 0.46) | 41.40 (32.91, 50.44) * | |
| Female | SO2 | 1.79 (0.05, 3.55) * | 2.67 (−1.79, 7.34) | 1.17 (−0.51, 2.87) | 15.34 (11.78, 19.02) * |
| PM10 | 0.37 (−0.17, 0.91) | 0.12 (−1.21, 1.46) | 0.34 (0.08, 0.60) * | 0.79 (−0.18, 1.78) | |
| NO2 | 0.85 (−2.92, 4.77) | 9.19 (−0.64, 20.01) | −3.67 (−8.19, 1.08) | 49.07 (33.02, 67.06) * | |
| Age | |||||
| <60 | SO2 | 0.47 (−0.62, 1.58) | 1.53 (−1.83, 5.00) | −0.81 (−1.95, 0.34) | 2.22 (0.34, 4.14)* |
| PM10 | 0.14 (−0.45, 0.74) | −0.87 (−2.59, 0.88) | 0.14 (−0.21, 0.49) | 0.56 (−0.41, 1.55) | |
| NO2 | 1.26 (−1.71, 4.31) | 1.65 (−6.91, 11.00) | −2.95 (−6.57, 0.81) | 9.02 (1.23, 17.40) * | |
| ≥60 | SO2 | 1.88 (0.51, 3.26) * | 6.96 (3.24, 10.81) * | 1.69 (0.35, 3.05) * | 20.51 (17.84, 23.24) * |
| PM10 | 0.53 (0.03, 1.02) * | 1.04 (−0.22, 2.32) | 0.33 (0.10, 0.56) * | 1.13 (0.25, 2.01) * | |
| NO2 | 2.39 (−0.99, 5.89) | 9.31 (0.34, 19.09) * | −0.79 (−4.55, 3.12) | 109.23 (92.57, 127.32) * | |
| Groups | Air Pollutants | TC | TG | HDL-C | LDL-C |
|---|---|---|---|---|---|
| % Change in GM (95% CI) | % Change in GM (95% CI) | % Change in GM (95% CI) | % Change in GM (95% CI) | ||
| warm | SO2 | 1.68 (0.37, 3.00) * | 7.93 (4.04, 11.96) * | 1.88 (0.46, 3.32) * | 24.11 (21.43, 26.85) * |
| PM10 | 0.05 (−0.16, 0.26) | 0.54 (−1.00, 2.10) | 0.23 (−0.02, 0.48) | 0.16 (−0.83, 1.15) | |
| NO2 | 2.62 (−0.43, 5.76) | 10.45 (1.481, 20.20) * | −2.05 (−5.61, 1.63) | 69.55 (57.91, 82.04) * | |
| cold | SO2 | 0.84 (−0.34, 2.03) | 1.58 (−1.70, 4.96) | −0.68 (−1.75, 0.40) | −0.86 (−2.64, 0.95) |
| PM10 | 0.31 (0.04, 0.58) * | 0.01 (−1.33, 1.36) | 0.34 (0.04, 0.65) * | 0.92 (0.17, 1.67) * | |
| NO2 | 0.47 (−3.08, 4.16) | 2.22 (−6.98, 12.33) | −2.77 (−6.55, 1.17) | 7.86 (−2.42, 19.22) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zheng, S.; Nie, Y.; Weng, J.; Cheng, N.; Hu, X.; Ren, X.; Pei, H.; Bai, Y. Association between Short-Term Exposure to Air Pollution and Dyslipidemias among Type 2 Diabetic Patients in Northwest China: A Population-Based Study. Int. J. Environ. Res. Public Health 2018, 15, 631. https://doi.org/10.3390/ijerph15040631
Wang M, Zheng S, Nie Y, Weng J, Cheng N, Hu X, Ren X, Pei H, Bai Y. Association between Short-Term Exposure to Air Pollution and Dyslipidemias among Type 2 Diabetic Patients in Northwest China: A Population-Based Study. International Journal of Environmental Research and Public Health. 2018; 15(4):631. https://doi.org/10.3390/ijerph15040631
Chicago/Turabian StyleWang, Minzhen, Shan Zheng, Yonghong Nie, Jun Weng, Ning Cheng, Xiaobin Hu, Xiaowei Ren, Hongbo Pei, and Yana Bai. 2018. "Association between Short-Term Exposure to Air Pollution and Dyslipidemias among Type 2 Diabetic Patients in Northwest China: A Population-Based Study" International Journal of Environmental Research and Public Health 15, no. 4: 631. https://doi.org/10.3390/ijerph15040631





